Experimental genetic crosses in tsetse flies of the livestock pathogen Trypanosoma congolense savannah
- PMID: 38178172
- PMCID: PMC10765672
- DOI: 10.1186/s13071-023-06105-4
Experimental genetic crosses in tsetse flies of the livestock pathogen Trypanosoma congolense savannah
Abstract
Background: In tropical Africa animal trypanosomiasis is a disease that has severe impacts on the health and productivity of livestock in tsetse fly-infested regions. Trypanosoma congolense savannah (TCS) is one of the main causative agents and is widely distributed across the sub-Saharan tsetse belt. Population genetics analysis has shown that TCS is genetically heterogeneous and there is evidence for genetic exchange, but to date Trypanosoma brucei is the only tsetse-transmitted trypanosome with experimentally proven capability to undergo sexual reproduction, with meiosis and production of haploid gametes. In T. brucei sex occurs in the fly salivary glands, so by analogy, sex in TCS should occur in the proboscis, where the corresponding portion of the developmental cycle takes place. Here we test this prediction using genetically modified red and green fluorescent clones of TCS.
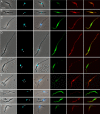
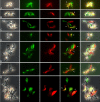
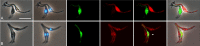
Methods: Three fly-transmissible strains of TCS were transfected with genes for red or green fluorescent protein, linked to a gene for resistance to the antibiotic hygromycin, and experimental crosses were set up by co-transmitting red and green fluorescent lines in different combinations via tsetse flies, Glossina pallidipes. To test whether sex occurred in vitro, co-cultures of attached epimastigotes of one red and one green fluorescent TCS strain were set up and sampled at intervals for 28 days.
Results: All interclonal crosses of genetically modified trypanosomes produced hybrids containing both red and green fluorescent proteins, but yellow fluorescent hybrids were only present among trypanosomes from the fly proboscis, not from the midgut or proventriculus. It was not possible to identify the precise life cycle stage that undergoes mating, but it is probably attached epimastigotes in the food canal of the proboscis. Yellow hybrids were seen as early as 14 days post-infection. One intraclonal cross in tsetse and in vitro co-cultures of epimastigotes also produced yellow hybrids in small numbers. The hybrid nature of the yellow fluorescent trypanosomes observed was not confirmed by genetic analysis.
Conclusions: Despite absence of genetic characterisation of hybrid trypanosomes, the fact that these were produced only in the proboscis and in several independent crosses suggests that they are products of mating rather than cell fusion. The three-way strain compatibility observed is similar to that demonstrated previously for T. brucei, indicating that a simple two mating type system does not apply for either trypanosome species.
Keywords: Green fluorescent protein; Mating; Red fluorescent protein; Sexual reproduction; Trypanosoma congolense; Tsetse fly.
© 2024. The Author(s).
Conflict of interest statement
No competing interests to declare.
Figures






Similar articles
-
Development of the livestock pathogen Trypanosoma (Nannomonas) simiae in the tsetse fly with description of putative sexual stages from the proboscis.Parasit Vectors. 2023 Jul 11;16(1):231. doi: 10.1186/s13071-023-05847-5. Parasit Vectors. 2023. PMID: 37434196 Free PMC article.
-
The life cycle of Trypanosoma (Nannomonas) congolense in the tsetse fly.Parasit Vectors. 2012 Jun 27;5:109. doi: 10.1186/1756-3305-5-109. Parasit Vectors. 2012. PMID: 22676292 Free PMC article.
-
Mating compatibility in the parasitic protist Trypanosoma brucei.Parasit Vectors. 2014 Feb 21;7:78. doi: 10.1186/1756-3305-7-78. Parasit Vectors. 2014. PMID: 24559099 Free PMC article.
-
Fluorescent proteins reveal what trypanosomes get up to inside the tsetse fly.Parasit Vectors. 2019 Jan 4;12(1):6. doi: 10.1186/s13071-018-3204-y. Parasit Vectors. 2019. PMID: 30609932 Free PMC article. Review.
-
Trypanosoma congolense: Molecular Toolkit and Resources for Studying a Major Livestock Pathogen and Model Trypanosome.Adv Parasitol. 2017;98:283-309. doi: 10.1016/bs.apar.2017.03.002. Epub 2017 May 5. Adv Parasitol. 2017. PMID: 28942771 Review.
References
-
- Programme Against African Trypanosomosis (PAAT). http://www.fao.org/ag/againfo/programmes/en/paat/disease.html.
-
- African Union: Interafrican Bureau for Animal Resources. http://www.au-ibar.org/index.php?option=com_flexicontent&view=items&cid=.... - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

