Prevention of symptomatic pulmonary embolism for gynecologic malignancies with preoperative asymptomatic venous thromboembolism: GOTIC-VTE trial
- PMID: 38178702
- PMCID: PMC11262890
- DOI: 10.3802/jgo.2024.35.e37
Prevention of symptomatic pulmonary embolism for gynecologic malignancies with preoperative asymptomatic venous thromboembolism: GOTIC-VTE trial
Abstract
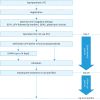
Objective: In Japan, perioperative prophylaxis of pulmonary embolism (PE) in gynecologic cancer patients with preoperative asymptomatic venous thromboembolism (VTE) has not been well established yet. The GOTIC-VTE trial was a prospective, multi-center, single-arm clinical trial to investigate the prevention of postoperative symptomatic PE onset by seamless anticoagulant therapy from the preoperative period to 4 weeks after surgery instead of using intermittent pneumatic compression.
Methods: Anticoagulant therapy was started immediately after asymptomatic VTE diagnosis and stopped preoperatively according to the rules of each institution. Unfractionated heparin administration was resumed within 12 hours postoperatively, and this was followed by the switch to low-molecular-weight heparin and subsequently, edoxaban; this cycle was continued for 28 days. Primary outcome was the occurrence of symptomatic PE in 28 days postoperatively. Secondary outcomes were the incidence of VTE-related events in 28 days and 6 months postoperatively and protocol-related adverse events.
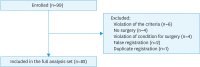
Results: Between February 2018 and September 2020, 99 patients were enrolled; of these, 82 patients were assessed as the full analysis set, including 58 for ovarian cancer, fallopian tube, or peritoneal cancer; 21 for endometrial cancer; and 3 for cervical cancer. No symptomatic PE was observed within 28 days postoperatively; two patients had bleeding events (major bleeding and clinically relevant nonmajor bleeding) and three had grade 3 adverse events (increased alanine transaminase, aspartate aminotransferase, or gamma-glutamyl transferase).
Conclusion: The multifaceted perioperative management for gynecologic malignancies with asymptomatic VTE effectively prevented postoperative symptomatic PE.
Trial registration: JRCT Identifier: jRCTs031180124.
Keywords: Anticoagulants; Gynecologic Neoplasms; Gynecologic Surgery; Pulmonary Embolism; Venous Thromboembolism.
© 2024. Asian Society of Gynecologic Oncology, Korean Society of Gynecologic Oncology, and Japan Society of Gynecologic Oncology.
Conflict of interest statement
YT, TS, and HF received honoraria from Daiichi Sankyo. KY received a research grant from Daiichi Sankyo. KH received honoraria and a research grant from Daiichi Sankyo. KF received honoraria from Daiichi Sankyo for consultation.
Figures
References
-
- Anderson FA, Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(Suppl 1):I9–16. - PubMed
-
- Nakamura M, Miyata T, Ozeki Y, Takayama M, Komori K, Yamada N, et al. Current venous thromboembolism management and outcomes in Japan. Circ J. 2014;78:708–717. - PubMed
-
- Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–722. - PubMed
-
- Walker AJ, Card TR, West J, Crooks C, Grainge MJ. Incidence of venous thromboembolism in patients with cancer - a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49:1404–1413. - PubMed
-
- Sørensen HT, Mellemkjaer L, Olsen JH, Baron JA. Prognosis of cancers associated with venous thromboembolism. N Engl J Med. 2000;343:1846–1850. - PubMed

