Antibodies to watch in 2024
- PMID: 38178784
- PMCID: PMC10773713
- DOI: 10.1080/19420862.2023.2297450
Antibodies to watch in 2024
Abstract
The 'Antibodies to Watch' article series provides an annual summary of commercially sponsored monoclonal antibody therapeutics currently in late-stage clinical development, regulatory review, and those recently granted a first approval in any country. In this installment, we discuss key details for 16 antibody therapeutics granted a first approval in 2023, as of November 17 (lecanemab (Leqembi), rozanolixizumab (RYSTIGGO), pozelimab (VEOPOZ), mirikizumab (Omvoh), talquetamab (Talvey), elranatamab (Elrexfio), epcoritamab (EPKINLY), glofitamab (COLUMVI), retifanlimab (Zynyz), concizumab (Alhemo), lebrikizumab (EBGLYSS), tafolecimab (SINTBILO), narlumosbart (Jinlitai), zuberitamab (Enrexib), adebrelimab (Arelili), and divozilimab (Ivlizi)). We briefly review 26 product candidates for which marketing applications are under consideration in at least one country or region, and 23 investigational antibody therapeutics that are forecast to enter regulatory review by the end of 2024 based on company disclosures. These nearly 50 product candidates include numerous innovative bispecific antibodies, such as odronextamab, ivonescimab, linvoseltamab, zenocutuzumab, and erfonrilimab, and antibody-drug conjugates, such as trastuzumab botidotin, patritumab deruxtecan, datopotamab deruxtecan, and MRG002, as well as a mixture of two immunocytokines (bifikafusp alfa and onfekafusp alfa). We also discuss clinical phase transition and overall approval success rates for antibody therapeutics, which are crucial to the biopharmaceutical industry because these rates inform decisions about resource allocation. Our analyses indicate that these molecules have approval success rates in the range of 14-32%, with higher rates associated with antibodies developed for non-cancer indications. Overall, our data suggest that antibody therapeutic development efforts by the biopharmaceutical industry are robust and increasingly successful.
Keywords: Antibody therapeutics; COVID-19; European Medicines Agency; Food and Drug Administration; SARS-CoV-2; cancer; immune-mediated disorders.
Conflict of interest statement
HK, LW, and JV are employed by companies that develop antibody therapeutics. SC and JMR are employed by The Antibody Society, a nonprofit trade association funded by corporate sponsors that develop antibody therapeutics or provide services to companies that develop antibody therapeutics. JMR is also Editor-in-Chief of
Figures
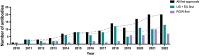
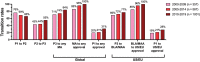
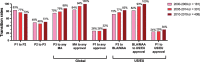

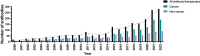
References
-
- Thomas D, Chancellor D, Micklus A, LaFever S, Hay M, Chaudhuri S, Bowden R, Low AW. Clinical development success rates and contributing factors 2011–2020. Biotechnology Innovation Organization Report; 2021. Feb. [accessed 2023 Sept 12]. https://www.bio.org/clinical-development-success-rates-and-contributing-....
-
- Thomas D, Burns J, Audette J, Carroll A, Dow-Hygelund C, Hay M. Clinical development success rates 2006-2015. Biotechnology Innovation Organization Report; 2016. [accessed 2023 Sept]. https://www.bio.org/sites/default/files/legacy/bioorg/docs/Clinical%20De....
-
- Eisai . FDA grants traditional approval for LEQEMBI® (lecanemab-irmb) for the treatment of Alzheimer’s disease; July 7, 2023, press release. [accessed 2023 Aug 15]. https://www.eisai.com/news/2023/news202349.html.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
