Evaluation of the humoral and mucosal immune response of a multiepitope vaccine against COVID-19 in pigs
- PMID: 38179057
- PMCID: PMC10765521
- DOI: 10.3389/fimmu.2023.1276950
Evaluation of the humoral and mucosal immune response of a multiepitope vaccine against COVID-19 in pigs
Abstract
Introduction: This study evaluated the immune response to a multiepitope recombinant chimeric protein (CHIVAX) containing B- and T-cell epitopes of the SARS-CoV-2 spike's receptor binding domain (RBD) in a translational porcine model for pre-clinical studies.
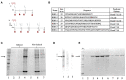
Methods: We generated a multiepitope recombinant protein engineered to include six coding conserved epitopes from the RBD domain of the SARS-CoV-2 S protein. Pigs were divided into groups and immunized with different doses of the protein, with serum samples collected over time to determine antibody responses by indirect ELISA and antibody titration. Peptide recognition was also analyzed by Western blotting. A surrogate neutralization assay with recombinant ACE2 and RBDs was performed. Intranasal doses of the immunogen were also prepared and tested on Vietnamese minipigs.
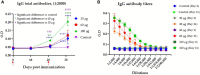
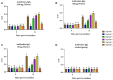
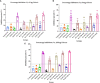
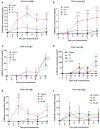
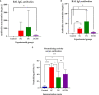
Results: When the immunogen was administered subcutaneously, it induced specific IgG antibodies in pigs, and higher doses correlated with higher antibody levels. Antibodies from immunized pigs recognized individual peptides in the multiepitope vaccine and inhibited RBD-ACE2 binding for five variants of concern (VOC). Comparative antigen delivery methods showed that both, subcutaneous and combined subcutaneous/intranasal approaches, induced specific IgG and IgA antibodies, with the subcutaneous approach having superior neutralizing activity. CHIVAX elicited systemic immunity, evidenced by specific IgG antibodies in the serum, and local mucosal immunity, indicated by IgA antibodies in saliva, nasal, and bronchoalveolar lavage secretions. Importantly, these antibodies demonstrated neutralizing activity against SARS-CoV-2 in vitro.
Discussion: The elicited antibodies recognized individual epitopes on the chimeric protein and demonstrated the capacity to block RBD-ACE2 binding of the ancestral SARS-CoV-2 strain and four VOCs. The findings provide proof of concept for using multiepitope recombinant antigens and a combined immunization protocol to induce a neutralizing immune response against SARS-CoV-2 in the pig translational model for preclinical studies.
Keywords: COVID-19; SARS-CoV-2; humoral response; mucosal immunity; multiepitopic vaccine; recombinant protein.
Copyright © 2023 Mosqueda, Hernández-Silva, Vega-López, Vega-Rojas, Beltrán, Velasco-Elizondo, Ramírez-Estudillo, Fragoso-Saavedra, Pérez-Almeida, Hernández, Melgoza-González, Hinojosa-Trujillo, Mercado-Uriostegui, Mejía-López, Rivera-Ballesteros and García-Gasca.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- World Health Organization . Timeline of WHO’s response to COVID-19 (2020). Available at: https://www.who.int/news-room/detail/29-06-2020-covidtimeline (Accessed July 28, 2020).
-
- Zhu F-C, Guan X-H, Li Y-H, Huang J-Y, Jiang T, Hou L-H, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomized, double-blind, placebo-controlled, phase 2 trial. Lancet (2020) 396:479–88. doi: 10.1016/S0140-6736(20)31605-6 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

