Aggregate assembly of ferrocene functionalized indium-oxo clusters
- PMID: 38179516
- PMCID: PMC10762979
- DOI: 10.1039/d3sc05824g
Aggregate assembly of ferrocene functionalized indium-oxo clusters
Abstract
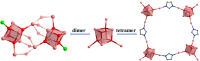
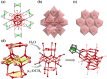
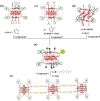
In this study, we synthesized multi-nuclear indium oxide clusters (InOCs) using 1,1'-ferrocene dicarboxylic acid (H2FcDCA) as the chelating and surface protection ligand. The obtained clusters include the cubane-type heptanuclear InOCs ([In7]) and the sandwich-type thirteen-nuclear InOCs ([In13]). Notably, [In13] represents the highest nuclear number reported within the InOC family. In addition, the presence of labile coordination sites in these clusters allowed for structural modification and self-assembly. A series of [In7] clusters with adjustable band gaps have been obtained and the self-assembly of [In7] clusters resulted in the formation of an Fe-doped dimer, [Fe2In12], and an imidazole-bridged tetramer, [In28]. Similarly, in the case of [In13] clusters, the coordinated water molecules could be replaced by imidazole, methylimidazole, and even a bridged carboxylic acid, allowing the construction of one-dimensional extended structures. Additionally, part of the H2FcDCA could be substituted by pyrazole. This flexibility in replacing solvent molecules offered diverse possibilities for tailoring the properties and structures of the InOCs to suit specific applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Al-Resheedi S. Alhokbany N. S. Mahfouz R. M. Mater. Res. 2015;18:931–938. doi: 10.1590/1516-1439.331814. - DOI
LinkOut - more resources
Full Text Sources

