Early COVID-19 vaccine effectiveness of XBB.1.5 vaccine against hospitalisation and admission to intensive care, the Netherlands, 9 October to 5 December 2023
- PMID: 38179623
- PMCID: PMC10905658
- DOI: 10.2807/1560-7917.ES.2024.29.1.2300703
Early COVID-19 vaccine effectiveness of XBB.1.5 vaccine against hospitalisation and admission to intensive care, the Netherlands, 9 October to 5 December 2023
Abstract
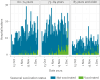
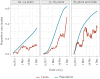
We present early vaccine effectiveness (VE) estimates of the 2023 seasonal COVID-19 XBB.1.5 vaccine against COVID-19 hospitalisation and admission to an intensive care unit (ICU) in previously vaccinated adults ≥ 60 years in the Netherlands. We compared vaccination status of 2,050 hospitalisations including 92 ICU admissions with age group-, sex-, region- and date-specific population vaccination coverage between 9 October and 5 December 2023. VE against hospitalisation was 70.7% (95% CI: 66.6-74.3), VE against ICU admission was 73.3% (95% CI: 42.2-87.6).
Keywords: COVID-19; COVID-19 vaccination; Vaccine effectiveness.
Conflict of interest statement
Figures
References
-
- National Institute for Public Health and the Environment (RIVM). Deelname COVID-19-vaccinatie in Nederland (19 december 2023) [Uptake of COVID-19 vaccination in the Netherlands (19 December 2023)]. Bilthoven: RIVM; 2023. [Dutch]. Available from: https://www.rivm.nl/corona/actueel/vaccinatiecijfers/update-deelname-cov...
-
- Government of the Netherlands. Coronavirus Dashboard. Virus particles in wastewater. The Hague: Rijksoverheid. [Accessed: 20 Dec 2023]. Available from: https://coronadashboard.government.nl/landelijk/rioolwater
-
- Government of the Netherlands. Coronavirus Dashboard. View on the hospitals. The Hague: Rijksoverheid. [Accessed: 12 Dec 2023]. Available from: https://coronadashboard.government.nl/landelijk/ziekenhuizen-in-beeld
-
- Van Werkhoven CH, De Gier B, McDonald SA, De Melker HE, Hahne SJM, Van den Hof S, et al. Information bias of vaccine effectiveness estimation due to informed consent for national registration of COVID-19 vaccination: estimation and correction using a data augmentation model. medRxiv. 2023:2023.05.23.23290384 .10.1101/2023.05.23.23290384 - DOI
-
- National Institute for Public Health and the Environment (RIVM). Uitvoeringsrichtlijn COVID-19-vaccinatie, versie 5.1 (8 december 2023). [COVID-19 guideline version 5.1 (8 December 2023)]. Bilthoven: RIVM; 2023. Dutch. Available from: https://lci.rivm.nl/richtlijnen/covid-19-vaccinatie
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
