Sox9 Accelerates Vascular Aging by Regulating Extracellular Matrix Composition and Stiffness
- PMID: 38179698
- PMCID: PMC10826924
- DOI: 10.1161/CIRCRESAHA.123.323365
Sox9 Accelerates Vascular Aging by Regulating Extracellular Matrix Composition and Stiffness
Abstract
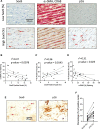
Background: Vascular calcification and increased extracellular matrix (ECM) stiffness are hallmarks of vascular aging. Sox9 (SRY-box transcription factor 9) has been implicated in vascular smooth muscle cell (VSMC) osteo/chondrogenic conversion; however, its relationship with aging and calcification has not been studied.
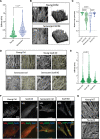
Methods: Immunohistochemistry was performed on human aortic samples from young and aged patients. Young and senescent primary human VSMCs were induced to produce ECM, and Sox9 expression was manipulated using adenoviral overexpression and depletion. ECM properties were characterized using atomic force microscopy and proteomics, and VSMC phenotype on hydrogels and the ECM were examined using confocal microscopy.
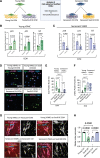
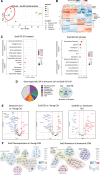
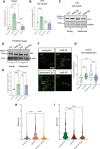
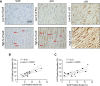
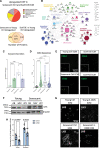
Results: In vivo, Sox9 was not spatially associated with vascular calcification but correlated with the senescence marker p16 (cyclin-dependent kinase inhibitor 2A). In vitro Sox9 showed mechanosensitive responses with increased expression and nuclear translocation in senescent cells and on stiff matrices. Sox9 was found to regulate ECM stiffness and organization by orchestrating changes in collagen (Col) expression and reducing VSMC contractility, leading to the formation of an ECM that mirrored that of senescent cells. These ECM changes promoted phenotypic modulation of VSMCs, whereby senescent cells plated on ECM synthesized from cells depleted of Sox9 returned to a proliferative state, while proliferating cells on a matrix produced by Sox9 expressing cells showed reduced proliferation and increased DNA damage, reiterating features of senescent cells. LH3 (procollagen-lysine, 2-oxoglutarate 5-dioxygenase 3) was identified as an Sox9 target and key regulator of ECM stiffness. LH3 is packaged into extracellular vesicles and Sox9 promotes extracellular vesicle secretion, leading to increased LH3 deposition within the ECM.
Conclusions: These findings highlight the crucial role of ECM structure and composition in regulating VSMC phenotype. We identify a positive feedback cycle, whereby cellular senescence and increased ECM stiffening promote Sox9 expression, which, in turn, drives further ECM modifications to further accelerate stiffening and senescence.
Keywords: atherosclerosis; calcium; extracellular matrix; extracellular vesicles; transcription factors.
Conflict of interest statement
Figures

Comment in
-
Aging Two-Step: SOX9's Influence on Vascular Stiffness and Senescence.Circ Res. 2024 Feb 2;134(3):325-327. doi: 10.1161/CIRCRESAHA.124.324212. Epub 2024 Feb 1. Circ Res. 2024. PMID: 38300983 Free PMC article. No abstract available.
References
-
- Bobryshev YV. Transdifferentiation of smooth muscle cells into chondrocytes in atherosclerotic arteries in situ: implications for diffuse intimal calcification. J Pathol. 2005;205:641–650. doi: 10.1002/path.1743 - PubMed
-
- Tyson KL, Reynolds JL, McNair R, Zhang Q, Weissberg PL, Shanahan CM. Osteo/chondrocytic transcription factors and their target genes exhibit distinct patterns of expression in human arterial calcification. Arterioscler Thromb Vasc Biol. 2003;23:489–494. doi: 10.1161/01.ATV.0000059406.92165.31 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

