Comparing Brain and Blood Lipidome Changes following Single and Repetitive Mild Traumatic Brain Injury in Rats
- PMID: 38179922
- PMCID: PMC10797623
- DOI: 10.1021/acschemneuro.3c00603
Comparing Brain and Blood Lipidome Changes following Single and Repetitive Mild Traumatic Brain Injury in Rats
Abstract
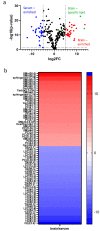
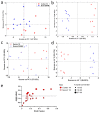
Traumatic brain injury (TBI) is a major health concern in the United States and globally, contributing to disability and long-term neurological problems. Lipid dysregulation after TBI is underexplored, and a better understanding of lipid turnover and degradation could point to novel biomarker candidates and therapeutic targets. Here, we investigated overlapping lipidome changes in the brain and blood using a data-driven discovery approach to understand lipid alterations in the brain and serum compartments acutely following mild TBI (mTBI) and the potential efflux of brain lipids to peripheral blood. The cortices and sera from male and female Sprague-Dawley rats were analyzed via ultra-high performance liquid chromatography-mass spectrometry (UHPLC-MS) in both positive and negative ion modes following single and repetitive closed head impacts. The overlapping lipids in the data sets were identified with an in-house data dictionary for investigating lipid class changes. MS-based lipid profiling revealed overall increased changes in the serum compartment, while the brain lipids primarily showed decreased changes. Interestingly, there were prominent alterations in the sphingolipid class in the brain and blood compartments after single and repetitive injury, which may suggest efflux of brain sphingolipids into the blood after TBI. Genetic algorithms were used for predictive panel selection to classify injured and control samples with high sensitivity and specificity. These overlapping lipid panels primarily mapped to the glycerophospholipid metabolism pathway with Benjamini-Hochberg adjusted q-values less than 0.05. Collectively, these results detail overlapping lipidome changes following mTBI in the brain and blood compartments, increasing our understanding of TBI-related lipid dysregulation while identifying novel biomarker candidates.
Keywords: Traumatic brain injury; blood biomarkers; brain biomarkers; lipidomics; ultra-high performance mass spectrometry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Integrative Analysis of Cytokine and Lipidomics Datasets Following Mild Traumatic Brain Injury in Rats.Metabolites. 2024 Feb 21;14(3):133. doi: 10.3390/metabo14030133. Metabolites. 2024. PMID: 38535293 Free PMC article.
-
Lipidome Alterations following Mild Traumatic Brain Injury in the Rat.Metabolites. 2022 Feb 5;12(2):150. doi: 10.3390/metabo12020150. Metabolites. 2022. PMID: 35208224 Free PMC article.
-
Discovery of Lipidome Alterations Following Traumatic Brain Injury via High-Resolution Metabolomics.J Proteome Res. 2018 Jun 1;17(6):2131-2143. doi: 10.1021/acs.jproteome.8b00068. Epub 2018 Apr 27. J Proteome Res. 2018. PMID: 29671324 Free PMC article.
-
Lipid profiling of brain tissue and blood after traumatic brain injury: A review of human and experimental studies.Semin Cell Dev Biol. 2021 Apr;112:145-156. doi: 10.1016/j.semcdb.2020.08.004. Epub 2020 Oct 7. Semin Cell Dev Biol. 2021. PMID: 33036880 Review.
-
[Mild traumatic brain injury and postconcussive syndrome: a re-emergent questioning].Encephale. 2012 Sep;38(4):329-35. doi: 10.1016/j.encep.2011.07.003. Epub 2011 Aug 31. Encephale. 2012. PMID: 22980474 Review. French.
Cited by
-
Near-Infrared Imaging of Glymphatic Clearance in a Pre-Clinical Model of Repetitive Closed Head Traumatic Brain Injury.Neurotrauma Rep. 2025 Jan 30;6(1):115-128. doi: 10.1089/neur.2024.0128. eCollection 2025. Neurotrauma Rep. 2025. PMID: 39990707 Free PMC article.
-
Valproic Acid Treatment after Traumatic Brain Injury in Mice Alleviates Neuronal Death and Inflammation in Association with Increased Plasma Lysophosphatidylcholines.Cells. 2024 Apr 23;13(9):734. doi: 10.3390/cells13090734. Cells. 2024. PMID: 38727269 Free PMC article.
-
Antioxidant and Anti-Inflammatory Properties of Melatonin in Secondary Traumatic Brain Injury.Antioxidants (Basel). 2024 Dec 28;14(1):25. doi: 10.3390/antiox14010025. Antioxidants (Basel). 2024. PMID: 39857359 Free PMC article. Review.
-
Integrative Analysis of Cytokine and Lipidomics Datasets Following Mild Traumatic Brain Injury in Rats.Metabolites. 2024 Feb 21;14(3):133. doi: 10.3390/metabo14030133. Metabolites. 2024. PMID: 38535293 Free PMC article.
References
-
- Dept. of Health and Human Services, Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths, 2002–2006. Faul M., Wald M. M., Xu L., Coronado V. G.,, Eds., 2010.
-
- Pu H. J.; Jiang X. Y.; Wei Z. S.; Hong D. D.; Hassan S.; Zhang W. T.; Liu J. L.; Meng H. X.; Shi Y. J.; Chen L.; et al. Repetitive and Prolonged Omega-3 Fatty Acid Treatment After Traumatic Brain Injury Enhances Long-Term Tissue Restoration and Cognitive Recovery. Cell Transplantation 2017, 26 (4), 555–569. 10.3727/096368916X693842. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

