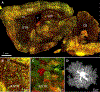
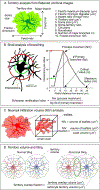
Astrocyte morphology
- PMID: 38180380
- PMCID: PMC11590062
- DOI: 10.1016/j.tcb.2023.09.006
Astrocyte morphology
Abstract
Astrocytes are predominant glial cells that tile the central nervous system (CNS). A cardinal feature of astrocytes is their complex and visually enchanting morphology, referred to as bushy, spongy, and star-like. A central precept of this review is that such complex morphological shapes evolved to allow astrocytes to contact and signal with diverse cells at a range of distances in order to sample, regulate, and contribute to the extracellular milieu, and thus participate widely in cell-cell signaling during physiology and disease. The recent use of improved imaging methods and cell-specific molecular evaluations has revealed new information on the structural organization and molecular underpinnings of astrocyte morphology, the mechanisms of astrocyte morphogenesis, and the contributions to disease states of reduced morphology. These insights have reignited interest in astrocyte morphological complexity as a cornerstone of fundamental glial biology and as a critical substrate for multicellular spatial and physiological interactions in the CNS.
Keywords: Sholl; electron microscopy; glia; imaging; morphology; neuropil; territory.
Copyright © 2023 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests The authors have no interests to declare.
Figures