Immunohistochemical field parcellation of the human hippocampus along its antero-posterior axis
- PMID: 38180568
- PMCID: PMC10917878
- DOI: 10.1007/s00429-023-02725-9
Immunohistochemical field parcellation of the human hippocampus along its antero-posterior axis
Abstract
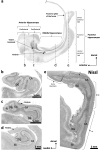
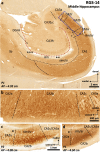
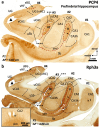
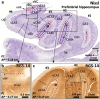
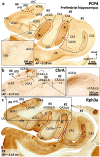
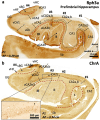
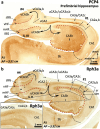
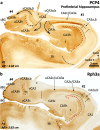
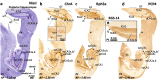
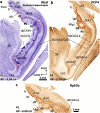
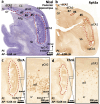
The primate hippocampus includes the dentate gyrus, cornu ammonis (CA), and subiculum. CA is subdivided into four fields (CA1-CA3, plus CA3h/hilus of the dentate gyrus) with specific pyramidal cell morphology and connections. Work in non-human mammals has shown that hippocampal connectivity is precisely patterned both in the laminar and longitudinal axes. One of the main handicaps in the study of neuropathological semiology in the human hippocampus is the lack of clear laminar and longitudinal borders. The aim of this study was to explore a histochemical segmentation of the adult human hippocampus, integrating field (medio-lateral), laminar, and anteroposterior longitudinal patterning. We provide criteria for head-body-tail field and subfield parcellation of the human hippocampus based on immunodetection of Rabphilin3a (Rph3a), Purkinje-cell protein 4 (PCP4), Chromogranin A and Regulation of G protein signaling-14 (RGS-14). Notably, Rph3a and PCP4 allow to identify the border between CA3 and CA2, while Chromogranin A and RGS-14 give specific staining of CA2. We also provide novel histological data about the composition of human-specific regions of the anterior and posterior hippocampus. The data are given with stereotaxic coordinates along the longitudinal axis. This study provides novel insights for a detailed region-specific parcellation of the human hippocampus useful for human brain imaging and neuropathology.
Keywords: Fasciola cinerea; Uncus; Andreas Retzius gyri; Band of Giacomini; Gyrus fasciolaris; Hippocampal body; Hippocampal head; Hippocampal tail; Hippocampus; Intralimbic gyrus; Parcellation; Posterior hippocampus; Segmentation; Vertical hippocampus.
© 2023. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures





Similar articles
-
Organization and Detailed Parcellation of Human Hippocampal Head and Body Regions Based on a Combined Analysis of Cyto- and Chemoarchitecture.J Comp Neurol. 2015 Oct 15;523(15):2233-53. doi: 10.1002/cne.23786. Epub 2015 Jul 15. J Comp Neurol. 2015. PMID: 25872498
-
Development of a histologically validated segmentation protocol for the hippocampal body.Neuroimage. 2017 Aug 15;157:219-232. doi: 10.1016/j.neuroimage.2017.06.008. Epub 2017 Jun 3. Neuroimage. 2017. PMID: 28587896
-
In vivo quantification of hippocampal subfields using 4.7 T fast spin echo imaging.Neuroimage. 2010 Jan 15;49(2):1224-30. doi: 10.1016/j.neuroimage.2009.09.042. Epub 2009 Sep 26. Neuroimage. 2010. PMID: 19786104
-
The CA2 hippocampal subfield in humans: A review.Hippocampus. 2023 Jun;33(6):712-729. doi: 10.1002/hipo.23547. Epub 2023 May 19. Hippocampus. 2023. PMID: 37204159 Review.
-
Psychosocial factors and hippocampal subfields: The Medea-7T study.Hum Brain Mapp. 2023 Apr 1;44(5):1964-1984. doi: 10.1002/hbm.26185. Epub 2022 Dec 30. Hum Brain Mapp. 2023. PMID: 36583397 Free PMC article.
Cited by
-
The fasciola cinereum of the hippocampal tail as an interventional target in epilepsy.Nat Med. 2024 May;30(5):1292-1299. doi: 10.1038/s41591-024-02924-9. Epub 2024 Apr 17. Nat Med. 2024. PMID: 38632391 Free PMC article.
-
Absolute Number of Three Populations of Interneurons and All GABAergic Synapses in the Human Hippocampus.J Neurosci. 2025 Mar 5;45(10):e0372242024. doi: 10.1523/JNEUROSCI.0372-24.2024. J Neurosci. 2025. PMID: 39809540
-
A Tail Yet to be Told: The Fasciola Cinereum in Epilepsy.Epilepsy Curr. 2025 Jan 17;25(2):125-127. doi: 10.1177/15357597241309175. eCollection 2025 Mar-Apr. Epilepsy Curr. 2025. PMID: 39845192 Free PMC article. No abstract available.
-
Mesoscale connectivity of the human hippocampus and fimbria revealed by ex vivo diffusion MRI.Neuroimage. 2025 Apr 15;310:121125. doi: 10.1016/j.neuroimage.2025.121125. Epub 2025 Mar 16. Neuroimage. 2025. PMID: 40101867 Free PMC article.
-
Sequence and trajectory of early Alzheimer's disease-related tau inclusions in the hippocampal formation of cases without amyloid-β deposits.Acta Neuropathol. 2025 May 23;149(1):50. doi: 10.1007/s00401-025-02862-x. Acta Neuropathol. 2025. PMID: 40407905 Free PMC article.
References
-
- Adickes ED, Folkerth RD, Sims KL. Use of perfusion fixation for improved neuropathologic examination. Arch Pathol Lab Med. 1997;121:1199–1206. - PubMed
-
- Adler DH, Wisse LEM, Ittyerah R, Pluta JB, Ding SL, Xie L, Wang J, Kadivar S, Robinson JL, Schuck T, Trojanowski JQ, Grossman M, Detre JA, Elliott MA, Toledo JB, Liu W, Pickup S, Miller MI, Das SR, Wolk DA, Yushkevich PA. Characterizing the human hippocampus in aging and Alzheimer's disease using a computational atlas derived from ex vivo MRI and histology. Proc Natl Acad Sci USA. 2018;115:4252–4257. doi: 10.1073/pnas.1801093115. - DOI - PMC - PubMed
-
- Annese J, Schenker-Ahmed NM, Bartsch H, Maechler P, Sheh C, Thomas N, Kayano J, Ghatan A, Bresler N, Frosch MP, Klaming R, Corkin S. Postmortem examination of patient H M 's brain based on histological sectioning and digital 3D reconstruction. Nat Commun. 2014;5:3122. doi: 10.1038/ncomms4122. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

