Assessment of mesh shrinkage using fibroblast-populated collagen matrices: a proof of concept for in vitro hernia mesh testing
- PMID: 38180627
- PMCID: PMC10997730
- DOI: 10.1007/s10029-023-02941-6
Assessment of mesh shrinkage using fibroblast-populated collagen matrices: a proof of concept for in vitro hernia mesh testing
Abstract
Purpose: This study uses free-floating contractile fibroblast-populated collagen matrices (FPCMs) to test the shrinkage of different hernia mesh products. We hope to present this model as a proof of concept for the development of in vitro hernia mesh testing-a novel technology with interesting potential.
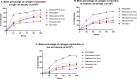
Methods: FPCMs were formed by seeding Human Dermal Fibroblasts into collagen gels. FPCMs were seeded with three different cell densities and cast at a volume of 500 μl into 24-well plates. Five different mesh products were embedded within the collagen constructs. Gels were left to float freely within culture media and contract over 5 days. Photographs were taken daily and the area of the collagen gel and mesh were measured. Media samples were taken at days 2 and 4 for the purposes of measuring MMP-9 release. After 5 days, dehydrated FPCMs were also examined under light and fluorescence microscopy to assess cell morphology.
Results: Two mesh products-the mosquito net and large pore lightweight mesh were found to shrink notably more than others. This pattern persisted across all three cell densities. There were no appreciable differences observed in MMP-9 release between products.
Conclusions: This study has successfully demonstrated that commercial mesh products can be successfully integrated into free-floating contractile FPCMs. Not only this, but FPCMs are capable of applying a contractile force upon those mesh products-eliciting different levels of contraction between mesh products. Such findings demonstrate this technique as a useful proof of concept for future development of in vitro hernia mesh testing.
Keywords: Hernia; In vitro; Materials; Mesh; Testing.
© 2024. The Author(s).
Conflict of interest statement
The authors have no other conflicts of interest.
Figures





Similar articles
-
Fibroblast matrix implants-a better alternative for incisional hernia repair?Biomed Mater. 2024 Apr 25;19(3). doi: 10.1088/1748-605X/ad3da4. Biomed Mater. 2024. PMID: 38604155
-
A simple combined floating and anchored collagen gel for enhancing mechanical strength of culture system.J Biomed Mater Res A. 2007 Jan;80(1):123-30. doi: 10.1002/jbm.a.30835. J Biomed Mater Res A. 2007. PMID: 16983652
-
Doxycycline alters collagen composition following ventral hernia repair.Surg Endosc. 2017 Apr;31(4):1659-1666. doi: 10.1007/s00464-016-5155-8. Epub 2016 Aug 12. Surg Endosc. 2017. PMID: 27519589
-
The lightweight and large porous mesh concept for hernia repair.Expert Rev Med Devices. 2005 Jan;2(1):103-17. doi: 10.1586/17434440.2.1.103. Expert Rev Med Devices. 2005. PMID: 16293033 Review.
-
A decade of ventral incisional hernia repairs with biologic acellular dermal matrix: what have we learned?Plast Reconstr Surg. 2012 Nov;130(5 Suppl 2):194S-202S. doi: 10.1097/PRS.0b013e318265a5ec. Plast Reconstr Surg. 2012. PMID: 23096971 Review.
References
-
- Idrees S, Jindal S, Gupta MK, Sarangi R. Surgical meshes—the search continues. Curr Med Res Pract. 2018;8:177–182. doi: 10.1016/j.cmrp.2018.08.005. - DOI
-
- Procter L, Falco EE, Fisher JP, Roth JS. Abdominal wall hernias and biomaterials. In: Gefen A, editor. Bioengineering research of chronic wounds: a multidisciplinary study approach. Berlin: Springer; 2009. pp. 425–447.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

