Network analysis of monoamines involved in anxiety-like behavior in a rat model of osteoarthritis
- PMID: 38180634
- PMCID: PMC10830664
- DOI: 10.1007/s43440-023-00562-5
Network analysis of monoamines involved in anxiety-like behavior in a rat model of osteoarthritis
Abstract
Background: Chronic pain is a major health problem that affects a significant number of patients, resulting in personal suffering and substantial health care costs. One of the most commonly reported causal conditions is osteoarthritis (OA). In addition to sensory symptoms, chronic pain shares an inherent overlap with mood or anxiety disorders. The involvement of the frontal cortex, striatum and nucleus accumbens, in the affective processing of pain is still poorly understood.
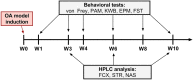
Methods: Male Wistar rats were divided into two groups: MIA (monoiodoacetate injected into the knee-model of OA) and sham (NaCl). Behavioral tests assessing pain, anxiety, and depressive behavior were performed at week 1, 3, 4, 6, 8, and 10. Neurochemical assays were conducted at weeks 3, 6, and 10 post-MIA injection, followed by the neurotransmitters and their metabolites correlation matrix and network analysis.
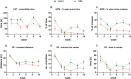
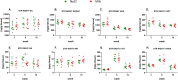
Results: OA animals developed rapid pain phenotype, whereas anxiety-like behavior accompanied the development of a pain phenotype from 6 week post-MIA injection. We did not detect any depressive-like behavior. Instead, immobility time measured in the forced swimming test transiently decreased at 3 weeks post-MIA in the OA group. We detected changes in noradrenaline and serotonin levels in analyzed structures at distinct time points. Network analysis revealed noradrenaline and serotonin neurotransmission changes in the nucleus accumbens, confirming it to be the key structure affected by chronic pain.
Conclusion: Animals with chronic pain exhibit symptoms of anxiety-like behavior and we identified underlying neurochemical changes using network analysis.
Keywords: Anxiety; Chronic pain; Depression; Monoamines; Nucleus accumbens; Osteoarthritis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Withdrawal from repeated administration of a low dose of reserpine induced opposing adaptive changes in the noradrenaline and serotonin system function: a behavioral and neurochemical ex vivo and in vivo studies in the rat.Prog Neuropsychopharmacol Biol Psychiatry. 2015 Mar 3;57:146-54. doi: 10.1016/j.pnpbp.2014.10.009. Epub 2014 Nov 8. Prog Neuropsychopharmacol Biol Psychiatry. 2015. PMID: 25445479
-
[Neurochemical mechanisms of depression-like behavior in WAG/Rij rats].Zh Vyssh Nerv Deiat Im I P Pavlova. 2013 May-Jun;63(3):303-15. doi: 10.7868/s0044467713030106. Zh Vyssh Nerv Deiat Im I P Pavlova. 2013. PMID: 24450162 Russian.
-
Evidence For Cannabidiol Modulation of Serotonergic Transmission in a Model of Osteoarthritis via in vivo PET Imaging and Behavioral Assessment.Int J Innov Res Med Sci. 2022 Jun;7(6):254-271. doi: 10.23958/ijirms/vol07-i06/1418. Epub 2022 Jun 3. Int J Innov Res Med Sci. 2022. PMID: 37841504 Free PMC article.
-
Inhibition of anandamide breakdown reduces pain and restores LTP and monoamine levels in the rat hippocampus via the CB1 receptor following osteoarthritis.Neuropharmacology. 2023 Jan 1;222:109304. doi: 10.1016/j.neuropharm.2022.109304. Epub 2022 Oct 28. Neuropharmacology. 2023. PMID: 36341807 Review.
-
The Pharmacology of Pain Associated With the Monoiodoacetate Model of Osteoarthritis.Front Pharmacol. 2019 Sep 18;10:974. doi: 10.3389/fphar.2019.00974. eCollection 2019. Front Pharmacol. 2019. PMID: 31619987 Free PMC article. Review.
Cited by
-
Widespread and Heterologous Effects of L-DOPA on Monoaminergic Tissue Metabolism in Newborn Rats Expressing Air-Stepping.Int J Mol Sci. 2025 Mar 5;26(5):2298. doi: 10.3390/ijms26052298. Int J Mol Sci. 2025. PMID: 40076918 Free PMC article.
References
-
- Bryk M, Chwastek J, Mlost J, Kostrzewa M, Starowicz K. Sodium monoiodoacetate dose-dependent changes in matrix metalloproteinases and inflammatory components as prognostic factors for the progression of osteoarthritis. Front Pharmacol. 2021;12:643605. doi: 10.3389/fphar.2021.643605. - DOI - PMC - PubMed
-
- Aqeel M, Rehna T, Sarfraz R. The association among perception of osteoarthritis with adverse pain anxiety, symptoms of depression, positive and negative affects in patients with knee osteoarthritis: a cross sectional study. J Pak Med Assoc. 2021;71(2(B)):645–650. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
