Weight Changes and Adverse Pregnancy Outcomes With Dolutegravir- and Tenofovir Alafenamide Fumarate-Containing Antiretroviral Treatment Regimens During Pregnancy and Postpartum
- PMID: 38180851
- PMCID: PMC11175665
- DOI: 10.1093/cid/ciae001
Weight Changes and Adverse Pregnancy Outcomes With Dolutegravir- and Tenofovir Alafenamide Fumarate-Containing Antiretroviral Treatment Regimens During Pregnancy and Postpartum
Abstract
Background: We evaluated associations between antepartum weight change and adverse pregnancy outcomes and between antiretroviral therapy (ART) regimens and week 50 postpartum body mass index in IMPAACT 2010.
Methods: Women with human immunodeficiency virus (HIV)-1 in 9 countries were randomized 1:1:1 at 14-28 weeks' gestational age (GA) to start dolutegravir (DTG) + emtricitabine (FTC)/tenofovir alafenamide fumarate (TAF) versus DTG + FTC/tenofovir disoproxil fumarate (TDF) versus efavirenz (EFV)/FTC/TDF. Insufficient antepartum weight gain was defined using Institute of Medicine guidelines. Cox-proportional hazards regression models were used to evaluate the association between antepartum weight change and adverse pregnancy outcomes: stillbirth (≥20 weeks' GA), preterm delivery (<37 weeks' GA), small size for GA (<10th percentile), and a composite of these endpoints.
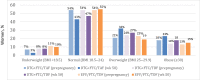
Results: A total of 643 participants were randomized: 217 to the DTG + FTC/TAF, 215 to the DTG + FTC/TDF, and 211 to the EFV/FTC/TDF arm. Baseline medians were as follows: GA, 21.9 weeks; HIV RNA, 903 copies/mL; and CD4 cell count, 466/μL. Insufficient weight gain was least frequent with DTG + FTC/TAF (15.0%) versus DTG + FTC/TDF (23.6%) and EFV/FTC/TDF (30.4%). Women in the DTG + FTC/TAF arm had the lowest rate of composite adverse pregnancy outcome. Low antepartum weight gain was associated with higher hazard of composite adverse pregnancy outcome (hazard ratio, 1.44 [95% confidence interval, 1.04-2.00]) and small size for GA (1.48 [.99-2.22]). More women in the DTG + FTC/TAF arm had a body mass index ≥25 (calculated as weight in kilograms divided by height in meters squared) at 50 weeks postpartum (54.7%) versus the DTG + FTC/TDF (45.2%) and EFV/FTC/TDF (34.2%) arms.
Conclusions: Antepartum weight gain on DTG regimens was protective against adverse pregnancy outcomes typically associated with insufficient weight gain, supportive of guidelines recommending DTG-based ART for women starting ART during pregnancy. Interventions to mitigate postpartum weight gain are needed.
Keywords: HIV; adverse pregnancy outcomes; antepartum weight change; postpartum weight; women's health.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. R. M. H. is an Elsevier ClinicalKey editorial board member. R. M. H. also reports honoraria for lectures on this topic from the Los Angeles County Division of HIV and STI Programs and the UCLA CARE Center’s continuing medical education seminars. S. B. and L. Z. reports support for the present work from the NIH Division of AIDS/Eunice Kennedy Shriver National Institute of Child Health and Human Development and ViiV/GSK, paid to their institution. L. F. reports grants or contracts for the current study through NIH/IMPAACT. S. H. reports grants or contracts from UKZN Developing Research Innovation, Localisation and Leadership in South Africa (DRILL), the Fogarty International Center, the NIH Common Fund, Office of Strategic Coordination, Office of the Director, Office of AIDS Research, the NIH Office of the Director, the National Institute of Mental Health, NIH (award D43TW010131, paid to their institution, from January 2018 to July 2023), and the SA National Research Foundation (Thuthuka funding grant, paid to their institution, from January 2019 to December 2021). R. Z. reports receipt of study medication from ViiV healthcare for a federally funded research study (as principal investigator); ViiV is donating long-acting cabotegravir for an implementation study of preexposure prophylaxis in Botswana, funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. J. S. C. is a scientific advisor for Merck, participating on its advisory board, and reports royalties or licenses from UpToDate. P. S. reports grants or contracts from Gilead and ViiV; consulting fees from Gilead, Janssen, Merck, and ViiV; and participation on a data safety monitoring board or advisory board for Merck. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
-
- McCann K, Moorhouse M, Sokhela S, et al. The ADVANCE clinical trial: changes from baseline to week 96 in DXA-assessed body composition in TAF/FTC + DTG compared to TDF/FTC + DTG, and TDF/FTC/EFV. In: 17th European AIDS Conference, November 6–9, 2019, Basel. Available at: https://www.natap.org/2019/EACS/EACS_31.htm. Accessed 12 May 2023.
-
- Venter WDF, Sokhela S, Simmons B, et al. Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): week 96 results from a randomised, phase 3, non-inferiority trial. Lancet HIV 2020; 7:e666–76. - PubMed
-
- Venter WDF, Bosch B, Sokhela S, et al. Final week 192 results from the ADVANCE trial: first-line TAF/FTC + DTG, TDF/FTC + DTG vs TDF/FTC/EFV. In: AIDS 2022—The 24th International AIDS Conference. Montreal, Canada, 2022. Available at: https://programme.aids2022.org/Abstract/Abstract/?abstractid=12600. Accessed 15 May 2023.
-
- UNAIDS . In danger: UNAIDS global AIDS update 2022. Available at: https://www.unaids.org/en/resources/documents/2022/in-danger-global-aids.... Accessed 3 August 2023.
Publication types
MeSH terms
Substances
Grants and funding
- UM1 AI069423/AI/NIAID NIH HHS/United States
- UM1 AI069456/AI/NIAID NIH HHS/United States
- U01 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI069399/AI/NIAID NIH HHS/United States
- UM1 AI069453/AI/NIAID NIH HHS/United States
- UM1 AI069436/AI/NIAID NIH HHS/United States
- K24 AI131928/AI/NIAID NIH HHS/United States
- U01 AI068632/AI/NIAID NIH HHS/United States
- MH/NIMH NIH HHS/United States
- UM1 AI068632/AI/NIAID NIH HHS/United States
- UM1 AI069469/AI/NIAID NIH HHS/United States
- UM1 AI069530/AI/NIAID NIH HHS/United States
- UM1 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI068616/AI/NIAID NIH HHS/United States
- UM1AI068632/NH/NIH HHS/United States
- UM1 AI106716/AI/NIAID NIH HHS/United States
- U01 AI069436/AI/NIAID NIH HHS/United States

