Statistical analysis of the storage time of finished product infusion
- PMID: 38180889
- PMCID: PMC10771069
- DOI: 10.1177/03000605231222231
Statistical analysis of the storage time of finished product infusion
Abstract
Objective: This retrospective study determined the storage time of finished infusion in each hospital ward and assessed whether the storage time of finished infusion was within an acceptable range.
Methods: The research object was the finished infusion (one bag of infusion with only one drug) that is centrally dosed at the Pharmacy Intravenous Admixture Service (PIVAS) of Jiading District Central Hospital Affiliated Shanghai University of Medicine & Health Sciences. We used an automatic scanner to assess the placement time of finished infusion products in various wards of the hospital. We classified the drugs used in various wards, analyzed whether their placement times were reasonable, assessed the reasons for unreasonable placement times, and took intervention measures. Similarly, the storage time of finished infusion was deemed reasonable or unreasonable, the reasons for unreasonable storage times were analyzed, and intervention measures were taken.
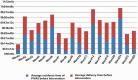
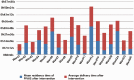
Results: In September 2021, the proportion of infusions stored for an unreasonable time was 12.69%, a decrease of 5.37% compared with August 2021, indicating the effectiveness of intervention measures.
Conclusion: By using statistical analysis and intervention measures, our PIVAS improved the standardized use of finished infusion products and ensured the safety of medication for patients.
Keywords: Pharmacy Intravenous Admixture Service; finished infusion; hospital ward; intravenous medicine; placement time; quality; storage time.
Conflict of interest statement
Declaration of conflicting interestsThe authors declare that they have no conflict of interest.
Figures
References
-
- Yang CS, Lin YZ, Zhang LL, et al.. Evidence-based evaluation of construction status of pharmacy intravenous admixture services in China [J]. Journal of Pediatric Pharmacy 2020; 26: 32–35.
-
- Huang J, Wu WY, Jiang ZF, et al.. Application of drug safety management system based on closed-loop concept [J]. Nursing Safety 2018; 18: 1103–1107.
-
- Yuan ZZ, Li QQ, Tang TT, et al.. Studies on the optimization of decontamination protocol for surfaces contaminated with cytotoxic drugs in PIVAS [J]. J Oncol Pharm Pract 2022; 12. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

