Global adoption of 6-month drug-resistant TB regimens: Projected uptake by 2026
- PMID: 38180980
- PMCID: PMC10769048
- DOI: 10.1371/journal.pone.0296448
Global adoption of 6-month drug-resistant TB regimens: Projected uptake by 2026
Erratum in
-
Correction: Global adoption of 6-month drug-resistant TB regimens: Projected uptake by 2026.PLoS One. 2024 Jan 31;19(1):e0298250. doi: 10.1371/journal.pone.0298250. eCollection 2024. PLoS One. 2024. PMID: 38295059 Free PMC article.
Abstract
Background: The WHO has issued a call to action urging countries to accelerate the rollout of new WHO-recommended shorter all-oral treatment regimens for drug-resistant TB (DR-TB), which remains a public-health crisis. The all-oral, 6-month BPaL/M regimen comprises 3-4 drugs: pretomanid used in combination with bedaquiline and linezolid, with or without moxifloxacin. This regimen has been recommended by the WHO for use in DR-TB patients instead of ≥9-month (up to 24-month) regimens. This study aims to project this regimen's use, along with its components bedaquiline, pretomanid and linezolid, and other treatments for DR-TB globally through 2026. It is intended to guide global health stakeholders in planning and budgeting for DR-TB interventions. Projected usage could help estimate cost of the individual components of DR-TB regimens over time.
Methods: Semi-structured interviews were conducted with national TB programme participants in key countries to gather intelligence on established plans and targets for use of various DR-TB treatment regimens from 2023 to 2026. These data informed development of projections for the global use of regimens and drugs.
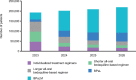
Results: Consistent global growth in the use of shorter regimens in DR-TB treatment was shown: BPaLM reaching 126,792 patients, BPaL reaching 43,716 patients, and the 9-11-month all-oral bedaquiline-based regimen reaching 13,119 patients by 2026. By 2026, the longer all-oral regimen is projected to be used by 19,262 patients, and individualised treatment regimens by 15,344 patients.
Conclusion: The study shows BPaL/M will be used in majority of DR-TB patients by 2024, reaching 78% by 2026. However, national efforts to scale-up, case-finding, monitoring, drug-susceptibility testing, and implementation of new treatments will be essential for ensuring they are accessible to all eligible patients in the coming years and goals for ending TB are met. There is an urgent need to engage communities in capacity building and demand generation.
Copyright: © 2024 Gupta et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
Authors, Aastha Gupta and Sandeep Juneja, are employed by TB Alliance, the non-profit product development partnership that developed pretomanid and the BPaL regimen for the treatment of drug-resistant tuberculosis. Author, Suvanand Sahu, serves on the Access Advisory Committee (an unpaid role) for TB Alliance, the non-profit product development partnership that developed pretomanid and the BPaL regimen for the treatment of drug-resistant tuberculosis. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



References
-
- World Health Organization. WHO urges countries to enable access to fully-oral drug-resistant TB treatment regimens. Geneva: World Health Organization, 2020. Available at: https://www.who.int/news-room/detail/15-06-2020-who-urges-countries-to-e... (accessed 18 April 2023).
-
- World Health Organization. Call to action: shorter and more effective treatment for all people suffering from drug-resistant TB. WHO/UCN/TB/2023.1. Geneva: World Health Organization, 2023. Available at: https://www.who.int/publications/m/item/call-to-action—shorter-an-d-more... (accessed 18 April 2023).
-
- Sharma A, Hill A, Kurbatova E, van der Walt M, Kvasnovsky C, Tupasi T, et al. Estimating the future burden of multidrug-resistant and extensively drug-resistant tuberculosis in India, the Philippines, Russia, and South Africa: a mathematical modelling study. Lancet Infect Dis 2017;17:707–15. doi: 10.1016/S1473-3099(17)30247-5 - DOI - PMC - PubMed
-
- General Assembly of the United Nations. Resolutions of the 73rd session. 2019. Available at: https://www.un.org/en/ga/73/resolutions.shtml (accessed 18 April 2023).
-
- General Assembly of the United Nations. Resolutions of the 73rd session. 2019. Available at: https://documents-dds-ny.un.org/doc/UNDOC/LTD/N23/272/22/PDF/N2327222.pdf (accessed 26 October 2023).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

