Pain-free oral delivery of biologic drugs using intestinal peristalsis-actuated microneedle robots
- PMID: 38181085
- PMCID: PMC10776013
- DOI: 10.1126/sciadv.adj7067
Pain-free oral delivery of biologic drugs using intestinal peristalsis-actuated microneedle robots
Abstract
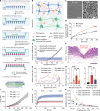
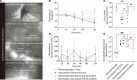
Biologic drugs hold immense promise for medical treatments, but their oral delivery remains a daunting challenge due to the harsh digestive environment and restricted gastrointestinal absorption. Here, inspired by the porcupinefish's ability to inflate itself and deploy its spines for defense, we proposed an intestinal microneedle robot designed to absorb intestinal fluids for rapid inflation and inject drug-loaded microneedles into the insensate intestinal wall for drug delivery. Upon reaching the equilibrium volume, the microneedle robot leverages rhythmic peristaltic contraction for mucosa penetration. The robot's barbed microneedles can then detach from its body during peristaltic relaxation and retain in the mucosa for drug releasing. Extensive in vivo experiments involving 14 minipigs confirmed the effectiveness of the intestinal peristalsis for microrobot actuation and demonstrated comparable insulin delivery efficacy to subcutaneous injection. The ingestible peristalsis-actuated microneedle robots may transform the oral administration of biologic drugs that primary relies on parenteral injection currently.
Figures



References
-
- Brown T. D., Whitehead K. A., Mitragotri S., Materials for oral delivery of proteins and peptides. Nat. Rev. Mater. 5, 127–148 (2020).
-
- Brayden D. J., Alonso M. J., Oral delivery of peptides: Opportunities and issues for translation. Adv. Drug Deliver. Rev. 106, 193–195 (2016). - PubMed
-
- Drucker D. J., Advances in oral peptide therapeutics. Nat. Rev. Drug Discov. 19, 277–289 (2020). - PubMed
-
- Wallace J. L., Granger D. N., The cellular and molecular basis of gastric mucosal defense. FASEB J. 10, 731–740 (1996). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

