Adverse Events Associated With Disease-Modifying Drugs for Multiple Sclerosis: A Multiregional Population-Based Study
- PMID: 38181306
- PMCID: PMC11097763
- DOI: 10.1212/WNL.0000000000208006
Adverse Events Associated With Disease-Modifying Drugs for Multiple Sclerosis: A Multiregional Population-Based Study
Abstract
Background and objectives: It is not possible to fully establish the safety of a disease-modifying drug (DMD) for multiple sclerosis (MS) from randomized controlled trials as only very common adverse events occurring over the short-term can be captured, and the quality of reporting has been variable. We examined the relationship between the DMDs for MS and potential adverse events in a multiregion population-based study.
Methods: We identified people with MS using linked administrative health data from 4 Canadian provinces. MS cases were followed from the most recent of first MS or related demyelinating disease event on January 1, 1996, until the earliest of emigration, death, or December 31, 2017. DMD exposure primarily comprised β-interferon, glatiramer acetate, natalizumab, fingolimod, dimethyl fumarate, teriflunomide, and alemtuzumab. We examined associations between DMD exposure and infection-related hospitalizations and physician visits using recurrent events proportional means models and between DMD exposure and 15 broad categories of incident adverse events using stratified multivariate Cox proportional hazard models.
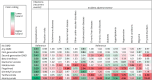
Results: We identified 35,894 people with MS. While virtually all DMDs were associated with a 42%-61% lower risk of infection-related hospitalizations, there was a modest increase in infection-related physician visits by 10%-33% for select DMDs. For incident adverse events, most elevated risks involved a second-generation DMD, with alemtuzumab's hazard of thyroid disorders being 19.42 (95% CI 9.29-36.51), hypertension 4.96 (95% CI 1.78-13.84), and cardiovascular disease 3.72 (95% CI 2.12-6.53). Natalizumab's highest risk was for cardiovascular disease (adjusted hazard ratio [aHR] 1.61; 95% CI 1.24-2.10). For the oral DMDs, fingolimod was associated with higher hazards of cerebrovascular (aHR 2.04; 95% CI 1.27-3.30) and ischemic heart diseases (aHR 1.64; 95% CI 1.10-2.44) and hypertension (aHR 1.73; 95% CI 1.30-2.31); teriflunomide with higher hazards of thyroid disorders (aHR 2.30; 95% CI 1.11-4.74), chronic liver disease (aHR 1.94; 95% CI 1.19-3.18), hypertension (aHR 1.76; 95% CI 1.32-2.37), and hyperlipidemia (aHR 1.61; 95% CI 1.07-2.44); and from complementary analyses (in 1 province), dimethyl fumarate with acute liver injury (aHR 6.55; 95% CI 1.96-21.87).
Discussion: Our study provides an extensive safety profile of several different DMDs used to treat MS in the real-world setting. Our findings not only complement those observed in short-term clinical trials but also provide new insights that help inform the risk-benefit profile of the DMDs used to treat MS in clinical practice. The results of this study highlight the continued need for long-term, independent safety studies of the DMDs used to treat MS.
Classification of evidence: This study provides Class III evidence that for patients with MS, while DMD exposure reduces the risk of infection-related hospitalizations, there are increased risks of infection-related physician visits and incident adverse events for select DMDs.
Conflict of interest statement
The authors report no disclosures relevant to the manuscript. R.A. Marrie is a coinvestigator on the CanProCo study funded by Biogen & Roche Canada. Go to
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
