Extracorporeal Life Support Organization Registry International Report 2022: 100,000 Survivors
- PMID: 38181413
- PMCID: PMC10962646
- DOI: 10.1097/MAT.0000000000002128
Extracorporeal Life Support Organization Registry International Report 2022: 100,000 Survivors
Abstract
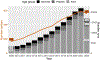
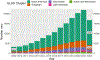
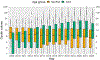
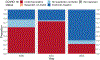
The Extracorporeal Life Support Organization (ELSO) maintains the world's largest extracorporeal membrane oxygenation (ECMO) registry by volume, center participation, and international scope. This 2022 ELSO Registry Report describes the program characteristics of ECMO centers, processes of ECMO care, and reported outcomes. Neonates (0-28 days), children (29 days-17 years), and adults (≥18 years) supported with ECMO from 2009 through 2022 and reported to the ELSO Registry were included. This report describes adjunctive therapies, support modes, treatments, complications, and survival outcomes. Data are presented descriptively as counts and percent or median and interquartile range (IQR) by year, group, or level. Missing values were excluded before calculating descriptive statistics. Complications are reported per 1,000 ECMO hours. From 2009 to 2022, 154,568 ECMO runs were entered into the ELSO Registry. Seven hundred and eighty centers submitted data during this time (557 in 2022). Since 2009, the median annual number of adult ECMO runs per center per year increased from 4 to 15, whereas for pediatric and neonatal runs, the rate decreased from 12 to 7. Over 50% of patients were transferred to the reporting ECMO center; 20% of these patients were transported with ECMO. The use of prone positioning before respiratory ECMO increased from 15% (2019) to 44% (2021) for adults during the coronavirus disease-2019 (COVID-19) pandemic. Survival to hospital discharge was greatest at 68.5% for neonatal respiratory support and lowest at 29.5% for ECPR delivered to adults. By 2022, the Registry had enrolled its 200,000th ECMO patient and 100,000th patient discharged alive. Since its inception, the ELSO Registry has helped centers measure and compare outcomes across its member centers and strategies of care. Continued growth and development of the Registry will aim to bolster its utility to patients and centers.
Copyright © ASAIO 2024.
Conflict of interest statement
Disclosure: J.E.T. is the Chair of the Registry Committee of the Extracorporeal Life Support Organization (ELSO). P.S.B. receives salary support from ELSO. G.M. is the President of ELSO. M.P. is the Immediate past President of ELSO. D.B. receives research support from and consults for LivaNova. He has been on the medical advisory boards for Abiomed, Xenios, Medtronic, Inspira, and Cellenkos. He is the President-elect of ELSO and the Chair of the Executive Committee of the International ECMO Network (ECMONet), and he writes for UpToDate. M.A., A.H., and K.R. are the Immediate Past Co-Chairs of the Scientific Oversight Committee of ELSO. P.R. is the Executive Director of ELSO. C.S. is the Chief Executive Officer (CEO) of ELSO. N.A.B. is the President of European Chapter of ELSO. N.A.B. has been on the medical advisory boards for Xenios and Baxter. T.M. is on the Board of Directors of ELSO. R.D.G. is the President of the Latin-American Chapter of ELSO. P.M.K. is the President of the South West Asia and Africa Chapter of ELSO. J.F.F. is the President of Asia-Pacific Chapter of ELSO. P.M.A.A. is Treasurer of ELSO Board of Directors. P.M.A.A. is funded by U.S. DoD PRMRP Clinical Trial Award #W81XWH2210301, NIH (R13HD104432) and FDA UCSF-Stanford Center of Excellence in Regulatory Sciences and Innovation (U01FD004979/U01FD005978). None of the funding sources were involved in the design or conduct of the study, collection, management, analysis, or interpretation of the data, or preparation, review, or approval of the manuscript. No other conflicts of interest reported. R.P.B. is a member of the Board of Directors for ELSO and receives funding from the National Heart, Lung, And Blood Institute (R01 HL153519).
Figures



Comment in
-
ELSO Registry Reports: A New Look.ASAIO J. 2024 Feb 1;70(2):144-145. doi: 10.1097/MAT.0000000000002145. Epub 2024 Jan 26. ASAIO J. 2024. PMID: 38289567 No abstract available.
References
-
- Toomasian JM, Snedecor SM, Cornell RG, Cilley RE, Bartlett RH: National experience with extracorporeal membrane oxygenation for newborn respiratory failure Data from 715 cases. ASAIO Trans 34: 140–147, 1988. - PubMed
-
- Stolar CJ, Delosh T, Bartlett RH: Extracorporeal life support organization 1993. ASAIO J 39: 976–979, 1993. - PubMed
-
- UK Collaborative Randomised Trial of Neonatal Extracorporeal Membrane Oxygenation: UK Collaborative ECMO Trail Group. Lancet 348: 75–82, 1996. - PubMed
-
- Mugford M, Elbourne D, Field D: Extracorporeal membrane oxygenation for severe respiratory failure in newborn infants. Cochrane Database Syst Rev (3): CD001340, 2008. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

