The role of cellular senescence in profibrillatory atrial remodelling associated with cardiac pathology
- PMID: 38181429
- PMCID: PMC11060482
- DOI: 10.1093/cvr/cvae003
The role of cellular senescence in profibrillatory atrial remodelling associated with cardiac pathology
Abstract
Aims: Cellular senescence is a stress-related or aging response believed to contribute to many cardiac conditions; however, its role in atrial fibrillation (AF) is unknown. Age is the single most important determinant of the risk of AF. The present study was designed to (i) evaluate AF susceptibility and senescence marker expression in rat models of aging and myocardial infarction (MI), (ii) study the effect of reducing senescent-cell burden with senolytic therapy on the atrial substrate in MI rats, and (iii) assess senescence markers in human atrial tissue as a function of age and the presence of AF.
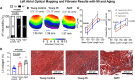
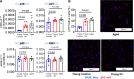
Methods and results: AF susceptibility was studied with programmed electrical stimulation. Gene and protein expression was evaluated by immunoblot or immunofluorescence (protein) and digital polymerase chain reaction (PCR) or reverse transcriptase quantitative PCR (messenger RNA). A previously validated senolytic combination, dasatinib and quercetin, (D+Q; or corresponding vehicle) was administered from the time of sham or MI surgery through 28 days later. Experiments were performed blinded to treatment assignment. Burst pacing-induced AF was seen in 100% of aged (18-month old) rats, 87.5% of young MI rats, and 10% of young control (3-month old) rats (P ≤ 0.001 vs. each). Conduction velocity was slower in aged [both left atrium (LA) and right atrium (RA)] and young MI (LA) rats vs. young control rats (P ≤ 0.001 vs. each). Atrial fibrosis was greater in aged (LA and RA) and young MI (LA) vs. young control rats (P < 0.05 for each). Senolytic therapy reduced AF inducibility in MI rats (from 8/9 rats, 89% in MI vehicle, to 0/9 rats, 0% in MI D + Q, P < 0.001) and attenuated LA fibrosis. Double staining suggested that D + Q acts by clearing senescent myofibroblasts and endothelial cells. In human atria, senescence markers were upregulated in older (≥70 years) and long-standing AF patients vs. individuals ≤60 and sinus rhythm controls, respectively.
Conclusion: Our results point to a potentially significant role of cellular senescence in AF pathophysiology. Modulating cell senescence might provide a basis for novel therapeutic approaches to AF.
Keywords: AF susceptibility; Cellular senescence; Fibrosis; Myocardial infarction; Senolytic drugs.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: none declared
Figures






Comment in
-
Is ageing a modifiable risk factor for atrial fibrillation?Cardiovasc Res. 2024 Apr 30;120(5):440-442. doi: 10.1093/cvr/cvae040. Cardiovasc Res. 2024. PMID: 38408875 No abstract available.
References
-
- Nattel S, Shiroshita-Takeshita A, Cardin S, Pelletier P. Mechanisms of atrial remodeling and clinical relevance. Curr Opin Cardiol 2005;20:21–25. - PubMed
-
- Wetzel U, Hindricks G, Piorkowski C. Atrial fibrillation in the elderly. Minerva Med 2009;100:145–150. - PubMed
-
- Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res 2014;114:1453–1468. - PubMed
-
- Laredo M, Waldmann V, Khairy P, Nattel S. Age as a critical determinant of atrial fibrillation: a two-sided relationship. Can J Cardiol 2018;34:1396–1406. - PubMed
-
- Bhatia GS, Lip GY. Atrial fibrillation post-myocardial infarction: frequency, consequences, and management. Curr Heart Fail Rep 2004;1:149–155. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

