PINK1 restrains periodontitis-induced bone loss by preventing osteoclast mitophagy impairment
- PMID: 38181706
- PMCID: PMC10789640
- DOI: 10.1016/j.redox.2023.103023
PINK1 restrains periodontitis-induced bone loss by preventing osteoclast mitophagy impairment
Abstract
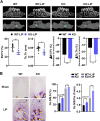
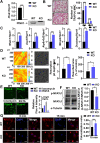
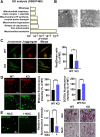
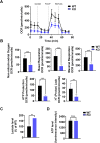
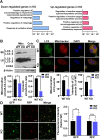
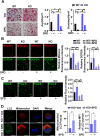
The oral colonization of periodontal pathogens onto gingival tissues establishes hypoxic microenvironment, often disrupting periodontal homeostasis in conjunction with oxidative stress. The association between reactive oxygen species (ROS) and osteolytic periodontitis have been suggested by recent studies. PTEN-induced kinase 1 (PINK1), a mitochondrial serine/threonine kinase, is an essential protein for mitochondrial quality control as it protects cells from oxidative stress by promoting degradation of damaged mitochondria through mitophagy. However, the pathophysiological roles of PINK1 in osteoclast-mediated bone loss have not been explored. Here we aimed to determine whether PINK1 plays a role in the regulation of osteoclastogenesis and alveolar bone resorption associated with periodontitis. C57BL/6 wild type (WT) and Pink1 knockout (KO) mice were subjected to ligature-induced periodontitis (LIP), and alveolar bones were evaluated by μCT-analysis and tartrate-resistant acid phosphatase (TRAP) staining. The μCT-analysis showed that bone volume fraction and travecular thickness were lower in Pink1 KO compared to WT mice. The number of TRAP-positive osteoclasts was markedly increased in the periodontal tissues of Pink1 KO mice with LIP. The genetic silencing or deletion of Pink1 promoted excessive osteoclast differentiation and bone resorption in vitro, as respectively indicated by TRAP staining and resorption pits on dentin slices. PINK1 deficiency led to mitochondrial instabilities as indicated by confocal microscopy of mitochondrial ROS, mitochondrial oxygen consumption rate (OCR) analysis, and transmission electron microscopy (TEM). Consequently, a significant increase in Ca2+-nuclear factor of activated T cells 1 (NFATc1) signaling was also found. On the other hand, restoration of mitophagy and autophagy by spermidine (SPD) treatment and the resolution of oxidative stress by N-acetyl-l-cysteine (NAC) treatment protected PINK1 deficiency-induced excessive generation of osteoclasts. Taken together, our findings demonstrate that PINK1 is essential for maintaining mitochondrial homeostasis during osteoclast differentiation. Therefore, targeting PINK1 may provide a novel therapeutic strategy for severe periodontitis with fulminant osteolysis.
Keywords: Mitophagy; Osteoclast; PINK1; Periodontitis; ROS.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Akalin F.A., Baltacioglu E., Alver A., Karabulut E. Lipid peroxidation levels and total oxidant status in serum, saliva and gingival crevicular fluid in patients with chronic periodontitis. J. Clin. Periodontol. 2007;34(7):558–565. - PubMed
-
- Sheikhi M., Gustafsson A., Jarstrand C. Cytokine, elastase and oxygen radical release by fusobacterium nucleatum-activated leukocytes: a possible pathogenic factor in periodontitis. J. Clin. Periodontol. 2000;27(10):758–762. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

