Hallmarks of stemness in mammalian tissues
- PMID: 38181752
- PMCID: PMC10769195
- DOI: 10.1016/j.stem.2023.12.006
Hallmarks of stemness in mammalian tissues
Abstract
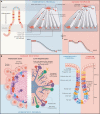
All adult tissues experience wear and tear. Most tissues can compensate for cell loss through the activity of resident stem cells. Although the cellular maintenance strategies vary greatly between different adult (read: postnatal) tissues, the function of stem cells is best defined by their capacity to replace lost tissue through division. We discuss a set of six complementary hallmarks that are key enabling features of this basic function. These include longevity and self-renewal, multipotency, transplantability, plasticity, dependence on niche signals, and maintenance of genome integrity. We discuss these hallmarks in the context of some of the best-understood adult stem cell niches.
Keywords: adult stem cells; hallmarks; lineage tracing; longevity; niche; organoids; plasticity; regeneration.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests H.C. and J.B. are inventors on patents held by the Royal Netherlands Academy of Arts and Sciences that cover organoid technology. H.C. is currently head of pharma Research and Early Development (pRED) at Roche, Basel, Switzerland. H.C.’s full disclosure is given at https://www.uu.nl/staff/JCClevers/.
Figures


References
-
- Post Y., Clevers H. Defining adult stem cell function at its simplest: the ability to replace lost cells through mitosis. Cell Stem Cell. 2019;25:174–183. - PubMed
-
- Clevers H., Watt F.M. Defining adult stem cells by function, not by phenotype. Annu. Rev. Biochem. 2018;87:1015–1027. - PubMed
-
- Knoblich J.A. Mechanisms of asymmetric stem cell division. Cell. 2008;132:P583–P597. - PubMed
-
- Loeffler D., Schroeder T. Symmetric and asymmetric activation of hematopoietic stem cells. Curr. Opin. Hematol. 2021;28:262–268. - PubMed
-
- Loeffler D., Wehling A., Schneiter F., Zhang Y., Müller-Bötticher N., Hoppe P.S., Hilsenbeck O., Kokkaliaris K.D., Endele M., Schroeder T. Asymmetric lysosome inheritance predicts activation of haematopoietic stem cells. Nature. 2019;573:426–429. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

