IELSG30 phase 2 trial: intravenous and intrathecal CNS prophylaxis in primary testicular diffuse large B-cell lymphoma
- PMID: 38181782
- PMCID: PMC10966164
- DOI: 10.1182/bloodadvances.2023011251
IELSG30 phase 2 trial: intravenous and intrathecal CNS prophylaxis in primary testicular diffuse large B-cell lymphoma
Abstract
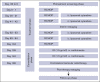
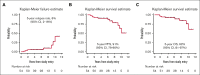
Primary testicular diffuse large B-cell lymphoma (PTL) is characterized by high risk of contralateral testis and central nervous system (CNS) relapse. Chemoimmunotherapy with intrathecal (IT) CNS prophylaxis and contralateral testis irradiation eliminates contralateral recurrences and reduces CNS relapses. The IELSG30 phase 2 study investigated feasibility and activity of an intensified IT and IV CNS prophylaxis. Patients with stage I/II PTL who had not received treatment received 2 cycles of IV high-dose methotrexate (MTX) (1.5 g/m2) after 6 cycles of the R-CHOP regimen (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone, every 21 days). IT liposomal cytarabine was administered on day 0 of cycles 2 to 5 of 21-day R-CHOP regimen. Contralateral testis radiotherapy (25-30 Gy) was recommended. Fifty-four patients (median age: 66 years) with stage I (n = 32) or II (n = 22) disease were treated with R-CHOP, 53 received at least 3 doses of IT cytarabine, 48 received at least 1 dose of IV MTX, and 50 received prophylactic radiotherapy. No unexpected toxicity occurred. At a median follow-up of 6 years, there was no CNS relapse; 7 patients progressed, and 8 died, with 5-year progression-free and overall survival rates of 91% (95% confidence interval [CI], 79-96) and 92% (95% CI, 81-97), respectively. Extranodal recurrence was documented in 6 patients (in 2 without nodal involvement). In 4 cases, the relapse occurred >6 years after treatment. Causes of death were lymphoma (n = 4), second primary malignancy (n = 1), cerebral vasculopathy (n = 1), unknown (n = 2). Intensive prophylaxis was feasible and effective in preventing CNS relapses. Late relapses, mainly at extranodal sites, represented the most relevant pattern of failure. This trial was registered at www.clinicaltrials.gov as #NCT00945724.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A. Conconi received speaker fees from Roche, AbbVie, Incyte, Takeda, and AstraZeneca, and was a member of advisory boards of Regeneron. A. Chiappella reports honoraria for lectures/educational activities from AstraZeneca, Bristol Myers Squibb/Celgene, Gilead-Sciences, Incyte, Janssen, Novartis, Roche, and Takeda, and was a member of advisory boards of Bristol Myers Squibb/Celgene, Gilead-Sciences, Ideogen, Janssen, Roche, Secura Bio, and Takeda. A.J.M.F. received speaker fees from Gilead and Roche; was a member of advisory boards of Gilead, Juno, Novartis, PletixaPharm, AstraZeneca, Bristol Myers Squibb, and Roche; currently receives research grants from ADC Therapeutics, Bayer HealthCare Pharmaceuticals, BeiGene, Bristol Myers Squibb, Genmab, Gilead, Hutchison Medipharma, Incyte, Janssen Research & Development, MEI Pharma, Novartis, PletixaPharm, Pharmacyclics, Protherics, Roche, and Takeda; and holds patents on NGR-hTNF in brain tumors and NGR-hTNF/R-CHOP in relapsed or refractory primary central nervous system lymphomas and SNGR-hTNF in brain tumors. A.S. reports research funding from AbbVie, ADC Therapeutics, Amgen, AstraZeneca, Bayer, Cellestia, Incyte, Loxo Oncology, Merck MSD, Novartis, Pfizer, Philogen, and Roche; consultancy honoraria from Roche, Janssen, Gilead, Bristol Myers Squibb/Celgene, and BeiGene; expert testimony for Bayer and Eli Lilly; and travel support from AstraZeneca and Incyte. G.G. is a member of advisory boards of AbbVie, AstraZeneca, BeiGene, Incyte, Janssen, and Roche, and is a member of the speaker’s bureau of Janssen. F.M. was a member of Takeda, Roche, Janssen, Gilead, MSD, Incyte, and Novartis, and reports that some travel expenses were covered by Takeda, Janssen, and Novartis. M.C.P. received a travel grant from BeiGene. U.V. is a member of advisory boards of Genmab, Incyte, AbbVie, and Gilead; lectures for Janssen, AbbVie, Incyte, and Roche; and was a research support for MorphoSys. E.Z. was a member of advisory boards of BeiGene, Bristol Myers Squibb, Curis, Eli/Lilly, Incyte, Janssen, Merck, Miltenyi Biomedicine, and Roche; received research support form AstraZeneca, BeiGene, Bristol Myers Squibb/Celgene, Incyte, Janssen, and Roche; and received travel grants from BeiGene, Janssen, Gilead, and Roche. The remaining authors declare no competing financial interests.
The current affiliation for F.E. is Hematology, Department of Biomedicine and Prevention, University Tor Vergata, Rome, Italy.
Figures
References
-
- Zucca E, Conconi A, Mughal TI, et al. Patterns of outcome and prognostic factors in primary large-cell lymphoma of the testis in a survey by the International Extranodal Lymphoma Study Group. J Clin Oncol. 2003;21(1):20–27. - PubMed
-
- Booman M, Douwes J, Glas AM, de Jong D, Schuuring E, Kluin PM. Primary testicular diffuse large B-cell lymphomas have activated B-cell-like subtype characteristics. J Pathol. 2006;210(2):163–171. - PubMed
-
- Deng L, Xu-Monette ZY, Loghavi S, et al. Primary testicular diffuse large B-cell lymphoma displays distinct clinical and biological features for treatment failure in rituximab era: a report from the International PTL Consortium. Leukemia. 2016;30(2):361–372. - PubMed
-
- King RL, Goodlad JR, Calaminici M, et al. Lymphomas arising in immune-privileged sites: insights into biology, diagnosis, and pathogenesis. Virchows Arch. 2020;476(5):647–665. - PubMed
-
- Kraan W, van Keimpema M, Horlings HM, et al. High prevalence of oncogenic MYD88 and CD79B mutations in primary testicular diffuse large B-cell lymphoma. Leukemia. 2014;28(3):719–720. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials