Acute stress facilitates habitual behavior in female rats
- PMID: 38181831
- PMCID: PMC10842801
- DOI: 10.1016/j.physbeh.2024.114456
Acute stress facilitates habitual behavior in female rats
Abstract
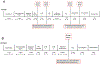
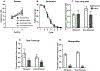
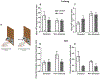
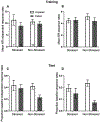
Instrumental behavior can reflect the influence of goal-directed and habitual systems. Contemporary research suggests that stress may facilitate control by the habitual system under conditions where the behavior would otherwise reflect control by the goal-directed system. However, it is unclear how stress modulates the influence of these systems on instrumental responding to achieve this effect, particularly in females. Here, we examine whether a mild psychogenic stressor experienced before acquisition training (Experiment 1), or prior to the test of expression (Experiment 2) would influence goal-directed and habitual control of instrumental responding in female rats. In both experiments, rats acquired an instrumental nose-poke response for a sucrose reward. This was followed by a reinforcer devaluation phase in which half the rats in Stressed and Non-Stressed conditions received pairings of the sucrose pellet with illness induced by lithium chloride until they rejected the pellet when offered. The remaining rats received a control treatment consisting of pellets and illness on separate days (Unpaired). Control by goal-directed and habitual systems was evaluated in a subsequent nonreinforced test of nose poking. The results of Experiment 1 indicated that the Non-Stressed Paired group reduced nose-poking compared to the Unpaired controls, identifying the response as goal directed, whereas the Stressed Paired and Unpaired groups made a similar number of nose pokes identifying the response as habitual despite a similar amount of training. Results from Experiment 2 indicated habitual control of nose-poke responding was present when stress was experienced just prior to the test. Collectively, these data suggest that stress may facilitate habitual control by altering the relative influence of goal-directed and habitual processes underpinning instrumental behavior. These results may be clinically relevant for understanding the contributions of stress to dysregulated instrumental behavior in compulsive pathologies.
Keywords: Action sequences; Females, Reinforcer devaluation; Goal-directed action; Habit; Stress.
Copyright © 2024. Published by Elsevier Inc.
Figures
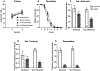
Similar articles
-
Dopamine sensitization by methamphetamine treatment prior to instrumental training delays the transition into habit in female rats.Behav Brain Res. 2022 Feb 10;418:113636. doi: 10.1016/j.bbr.2021.113636. Epub 2021 Oct 20. Behav Brain Res. 2022. PMID: 34687828
-
Inactivation of prelimbic and infralimbic cortex respectively affects minimally-trained and extensively-trained goal-directed actions.Neurobiol Learn Mem. 2018 Nov;155:164-172. doi: 10.1016/j.nlm.2018.07.010. Epub 2018 Jul 24. Neurobiol Learn Mem. 2018. PMID: 30053577 Free PMC article.
-
Female rats express habitual behavior earlier in operant training than males.Behav Neurosci. 2019 Feb;133(1):110-120. doi: 10.1037/bne0000282. Epub 2018 Oct 25. Behav Neurosci. 2019. PMID: 30359063
-
Stress-induced modulation of instrumental behavior: from goal-directed to habitual control of action.Behav Brain Res. 2011 Jun 1;219(2):321-8. doi: 10.1016/j.bbr.2010.12.038. Epub 2011 Jan 8. Behav Brain Res. 2011. PMID: 21219935 Review.
-
Context, attention, and the switch between habit and goal-direction in behavior.Learn Behav. 2021 Dec;49(4):349-362. doi: 10.3758/s13420-021-00488-z. Epub 2021 Oct 28. Learn Behav. 2021. PMID: 34713424 Free PMC article. Review.
Cited by
-
Estrous cycle stage gates the effect of stress on reward learning.Neuropsychopharmacology. 2025 Jul 16. doi: 10.1038/s41386-025-02170-8. Online ahead of print. Neuropsychopharmacology. 2025. PMID: 40670622
-
Goal-direction and habit in human and nonhuman behavioral sequences (behavior chains).J Exp Psychol Anim Learn Cogn. 2025 Apr;51(2):73-91. doi: 10.1037/xan0000395. J Exp Psychol Anim Learn Cogn. 2025. PMID: 40193516
-
Female rats retain goal-directed planning of action sequences after acute stress despite changes in planning structure and action sequence execution.Neurobiol Learn Mem. 2025 Jul;220:108063. doi: 10.1016/j.nlm.2025.108063. Epub 2025 May 15. Neurobiol Learn Mem. 2025. PMID: 40381721
References
-
- Adams CD (1982). Variations in the Sensitivity of Instrumental Responding to Reinforcer Devaluation. The Quarterly Journal of Experimental Psychology Section B, 34(2b), 77–98. 10.1080/14640748208400878 - DOI
-
- Adams CD, & Dickinson A (1981). Instrumental Responding following Reinforcer Devaluation. The Quarterly Journal of Experimental Psychology Section B, 33(2b), 109–121. 10.1080/14640748108400816 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

