BRCA1 mutation promotes sprouting angiogenesis in inflammatory cancer-associated fibroblast of triple-negative breast cancer
- PMID: 38182557
- PMCID: PMC10770063
- DOI: 10.1038/s41420-023-01768-5
BRCA1 mutation promotes sprouting angiogenesis in inflammatory cancer-associated fibroblast of triple-negative breast cancer
Abstract
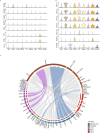
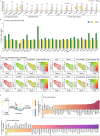
Triple-negative breast cancer (TNBC) is an aggressive breast cancer subtype with inferior outcomes owing to its low treatment response and high invasiveness. Based on abundant cancer-associated fibroblasts (CAFs) and frequent mutation of breast cancer-associated 1 (BRCA1) in TNBC, the characteristics of CAFs in TNBC patients with BRCA1 mutation compared to wild-type were investigated using single-cell analysis. Intriguingly, we observed that characteristics of inflammatory CAFs (iCAFs) were enriched in patients with BRCA1 mutation compared to the wild-type. iCAFs in patients with BRCA1 mutation exhibited outgoing signals to endothelial cells (ECs) clusters, including chemokine (C-X-C motif) ligand (CXCL) and vascular endothelial growth factor (VEGF). During CXCL signaling, the atypical chemokine receptor 1 (ACKR1) mainly interacts with CXCL family members in tumor endothelial cells (TECs). ACKR1-high TECs also showed high expression levels of angiogenesis-related genes, such as ANGPT2, MMP1, and SELE, which might lead to EC migration. Furthermore, iCAFs showed VEGF signals for FLT1 and KDR in TECs, which showed high co-expression with tip cell marker genes, including ZEB1 and MAFF, involved in sprouting angiogenesis. Moreover, BRCA1 mutation patients with relatively abundant iCAFs and tip cell gene expression exhibited a limited response to neoadjuvant chemotherapy, including cisplatin and bevacizumab. Importantly, our study observed the intricate link between iCAFs-mediated angiogenesis and chemoresistance in TNBC with BRCA1 mutation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Homologous recombination deficiency (HRD) status predicts response to standard neoadjuvant chemotherapy in patients with triple-negative or BRCA1/2 mutation-associated breast cancer.Breast Cancer Res Treat. 2018 Apr;168(3):625-630. doi: 10.1007/s10549-017-4624-7. Epub 2017 Dec 23. Breast Cancer Res Treat. 2018. PMID: 29275435
-
Incidence of BRCA1 somatic mutations and response to neoadjuvant chemotherapy in Chinese women with triple-negative breast cancer.Gene. 2016 Jun 10;584(1):26-30. doi: 10.1016/j.gene.2016.03.004. Epub 2016 Mar 5. Gene. 2016. PMID: 26956035
-
Tumor-Stroma-Inflammation Networks Promote Pro-metastatic Chemokines and Aggressiveness Characteristics in Triple-Negative Breast Cancer.Front Immunol. 2019 Apr 12;10:757. doi: 10.3389/fimmu.2019.00757. eCollection 2019. Front Immunol. 2019. PMID: 31031757 Free PMC article.
-
Biology and Management of Patients With Triple-Negative Breast Cancer.Oncologist. 2016 Sep;21(9):1050-62. doi: 10.1634/theoncologist.2016-0067. Epub 2016 Jul 11. Oncologist. 2016. PMID: 27401886 Free PMC article. Review.
-
Triple-negative breast cancer: disease entity or title of convenience?Nat Rev Clin Oncol. 2010 Dec;7(12):683-92. doi: 10.1038/nrclinonc.2010.154. Epub 2010 Sep 28. Nat Rev Clin Oncol. 2010. PMID: 20877296 Review.
Cited by
-
Resistance to neoadjuvant chemotherapy in breast cancers: a metabolic perspective.J Exp Clin Cancer Res. 2025 Aug 11;44(1):234. doi: 10.1186/s13046-025-03500-w. J Exp Clin Cancer Res. 2025. PMID: 40790759 Free PMC article. Review.
-
Revisiting the role of cancer-associated fibroblasts in tumor microenvironment.Front Immunol. 2025 Apr 17;16:1582532. doi: 10.3389/fimmu.2025.1582532. eCollection 2025. Front Immunol. 2025. PMID: 40313969 Free PMC article. Review.
-
Insights into the molecular and genetic role of obesity in breast cancer pathogenesis.Cancer Biol Ther. 2025 Dec;26(1):2501345. doi: 10.1080/15384047.2025.2501345. Epub 2025 May 12. Cancer Biol Ther. 2025. PMID: 40353441 Free PMC article. Review.
-
Decoding tumor-fibrosis interplay: mechanisms, impact on progression, and innovative therapeutic strategies.Front Pharmacol. 2024 Oct 23;15:1491400. doi: 10.3389/fphar.2024.1491400. eCollection 2024. Front Pharmacol. 2024. PMID: 39534084 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

