Preliminary study of the effect of low-intensity focused ultrasound on postpartum uterine involution and breast pain in puerperal women: a randomised controlled trial
- PMID: 38182657
- PMCID: PMC10770318
- DOI: 10.1038/s41598-024-51328-9
Preliminary study of the effect of low-intensity focused ultrasound on postpartum uterine involution and breast pain in puerperal women: a randomised controlled trial
Abstract
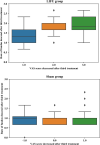
To evaluate the safety and efficacy of low-intensity focused ultrasound (LIFU) therapy in facilitating fundus descent and relieving postpartum breast pain compared with sham treatment. A multicentre, randomised, sham-controlled, blinded trial was conducted. A cohort of 176 eligible participants, who had normal prenatal check-ups and met the inclusion and exclusion criteria, were recruited from three medical centres and subsequently randomized into either the LIFU or sham group. All participants received three treatment sessions, wherein LIFU signal was applied to the uterus and breast sites using coupling gel, with the absence of ultrasound signal output in the sham group. Fundal height measurement and breast pain score were performed after each treatment. The primary outcome, uterine involution, was presented by measuring the fundal height of the uterus. The visual analogue scale (VAS) score, as a secondary outcome, was used to assess breast pain and determine the correlation between breast pain and fundal height as the outcome simultaneously. All participants were randomly assigned to either the LIFU group (n = 88) or sham group (n = 88), with seven individuals not completing the treatment. Overall, a statistically significant difference was noted in the rate and index of fundus descent after each treatment. The rate and index of fundus descent showed greater significance following the second treatment (rate: 1.5 (1.0, 2.0) cm/d; index: 0.15 (0.1, 0.18), P < 0.001) and third treatment (rate: 1.67 (1.33, 2.0) cm/d; index: 0.26 (0.23, 0.3), P < 0.001) in the LIFU group. VAS scores, which were based on the continuous variables for the baseline, first, second, and third treatments in the LIFU group (2.0 (2.0, 3.0), 1.0 (0.0, 2.0), 0.0 (0.0, 1.0), and 0.0 (0.0, 0.0) points, respectively), and the sham group (2.0 (2.0, 2.0), 2.0 (1.0, 2.0), 2.0 (1.0, 3.0), and 3.0 (1.0, 3.0) points, respectively), showed a statistically significant difference between the two groups. Meanwhile, the discrepancies in VAS score classification variables between the two groups were statistically significant. After the third treatment, a notable correlation was observed between the VAS score decrease and fundus descent rate; the more the VAS score decreased, the faster was the fundal decline rate in the LIFU group. LIFU therapy is safe and effective, contributing to the acceleration of uterine involution and the relief of postpartum breast pain.Trial ID The study has registered in the Chinese Clinical Trial Registry (ChiCTR2100049586) at 05/08/2021.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Grants and funding
- No.2023YFQ0070/Sichuan Province Science and Technology Support Program
- No.2023YFG0128/Sichuan Province Science and Technology Support Program
- No.2023NSFSC1606/Sichuan Province Science and Technology Support Program
- 2019YFS0008/Sichuan Province Science and Technology Support Program
- 2021YFC2009100/National Key Research and Development Program of China
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous