(Pro)renin receptor mediates tubular epithelial cell pyroptosis in diabetic kidney disease via DPP4-JNK pathway
- PMID: 38183100
- PMCID: PMC10768114
- DOI: 10.1186/s12967-023-04846-5
(Pro)renin receptor mediates tubular epithelial cell pyroptosis in diabetic kidney disease via DPP4-JNK pathway
Abstract
Background: (Pro)renin receptor (PRR) is highly expressed in renal tubules, which is involved in physiological and pathological processes. However, the role of PRR, expressed in renal tubular epithelial cells, in diabetic kidney disease (DKD) remain largely unknown.
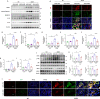
Methods: In this study, kidney biopsies, urine samples, and public RNA-seq data from DKD patients were used to assess PRR expression and cell pyroptosis in tubular epithelial cells. The regulation of tubular epithelial cell pyroptosis by PRR was investigated by in situ renal injection of adeno-associated virus9 (AAV9)-shRNA into db/db mice, and knockdown or overexpression of PRR in HK-2 cells. To reveal the underlined mechanism, the interaction of PRR with potential binding proteins was explored by using BioGrid database. Furthermore, the direct binding of PRR to dipeptidyl peptidase 4 (DPP4), a pleiotropic serine peptidase which increases blood glucose by degrading incretins under diabetic conditions, was confirmed by co-immunoprecipitation assay and immunostaining.
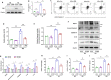
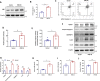
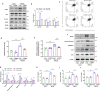
Results: Higher expression of PRR was found in renal tubules and positively correlated with kidney injuries of DKD patients, in parallel with tubular epithelial cells pyroptosis. Knockdown of PRR in kidneys significantly blunted db/db mice to kidney injury by alleviating renal tubular epithelial cells pyroptosis and the resultant interstitial inflammation. Moreover, silencing of PRR blocked high glucose-induced HK-2 pyroptosis, whereas overexpression of PRR enhanced pyroptotic cell death of HK-2 cells. Mechanistically, PRR selectively bound to cysteine-enrich region of C-terminal of DPP4 and augmented the protein abundance of DPP4, leading to the downstream activation of JNK signaling and suppression of SIRT3 signaling and FGFR1 signaling, and then subsequently mediated pyroptotic cell death.
Conclusions: This study identified the significant role of PRR in the pathogenesis of DKD; specifically, PRR promoted tubular epithelial cell pyroptosis via DPP4 mediated signaling, highlighting that PRR could be a promising therapeutic target in DKD.
Keywords: (Pro)renin receptor; Diabetic kidney disease; Dipeptidyl peptidase 4; Renal tubular cell pyroptosis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures




Similar articles
-
Targeting programmed cell death in diabetic kidney disease: from molecular mechanisms to pharmacotherapy.Mol Med. 2024 Dec 20;30(1):265. doi: 10.1186/s10020-024-01020-5. Mol Med. 2024. PMID: 39707216 Free PMC article. Review.
-
Sitagliptin ameliorates renal tubular injury in diabetic kidney disease via STAT3-dependent mitochondrial homeostasis through SDF-1α/CXCR4 pathway.FASEB J. 2020 Jun;34(6):7500-7519. doi: 10.1096/fj.201903038R. Epub 2020 Apr 12. FASEB J. 2020. PMID: 32281218
-
Elevation of ISG15 promotes diabetic kidney disease by modulating renal tubular epithelial cell pyroptosis.Clin Transl Med. 2025 Jun;15(6):e70337. doi: 10.1002/ctm2.70337. Clin Transl Med. 2025. PMID: 40462493 Free PMC article.
-
PDIA4 targets IRE1α/sXBP1 to alleviate NLRP3 inflammasome activation and renal tubular injury in diabetic kidney disease.Biochim Biophys Acta Mol Basis Dis. 2025 Mar;1871(3):167645. doi: 10.1016/j.bbadis.2024.167645. Epub 2024 Dec 30. Biochim Biophys Acta Mol Basis Dis. 2025. PMID: 39743023
-
Research Progress of Pyroptosis in Diabetic Kidney Disease.Int J Mol Sci. 2024 Jun 28;25(13):7130. doi: 10.3390/ijms25137130. Int J Mol Sci. 2024. PMID: 39000237 Free PMC article. Review.
Cited by
-
Slc25a21 in cisplatin-induced acute kidney injury: a new target for renal tubular epithelial protection by regulating mitochondrial metabolic homeostasis.Cell Death Dis. 2024 Dec 18;15(12):891. doi: 10.1038/s41419-024-07231-2. Cell Death Dis. 2024. PMID: 39695098 Free PMC article.
-
Aminopeptidasic Enzymes as Early Biomarkers of Cardiac Surgery-Associated Acute Kidney Injury and Long-Term Events.Biomolecules. 2024 Aug 24;14(9):1049. doi: 10.3390/biom14091049. Biomolecules. 2024. PMID: 39334816 Free PMC article.
-
The role of JNK signaling pathway in organ fibrosis.J Adv Res. 2025 Aug;74:207-223. doi: 10.1016/j.jare.2024.09.029. Epub 2024 Oct 2. J Adv Res. 2025. PMID: 39366483 Free PMC article. Review.
-
Targeting programmed cell death in diabetic kidney disease: from molecular mechanisms to pharmacotherapy.Mol Med. 2024 Dec 20;30(1):265. doi: 10.1186/s10020-024-01020-5. Mol Med. 2024. PMID: 39707216 Free PMC article. Review.
-
Epithelial DPP4 promotes Ang II-driven renal fibrosis by targeting ACE2 activity in the renin-angiotensin system.Int J Biol Sci. 2025 Jun 9;21(9):3901-3916. doi: 10.7150/ijbs.106418. eCollection 2025. Int J Biol Sci. 2025. PMID: 40607249 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

