Systematic engineering enables efficient biosynthesis of L-phenylalanine in E. coli from inexpensive aromatic precursors
- PMID: 38183119
- PMCID: PMC10768146
- DOI: 10.1186/s12934-023-02282-0
Systematic engineering enables efficient biosynthesis of L-phenylalanine in E. coli from inexpensive aromatic precursors
Erratum in
-
Correction: Systematic engineering enables efficient biosynthesis of L-phenylalanine in E. Coli from inexpensive aromatic precursors.Microb Cell Fact. 2024 Feb 10;23(1):46. doi: 10.1186/s12934-024-02327-y. Microb Cell Fact. 2024. PMID: 38341598 Free PMC article. No abstract available.
Abstract
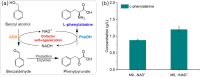
Background: L-phenylalanine is an essential amino acid with various promising applications. The microbial pathway for L-phenylalanine synthesis from glucose in wild strains involves lengthy steps and stringent feedback regulation that limits the production yield. It is attractive to find other candidates, which could be used to establish a succinct and cost-effective pathway for L-phenylalanine production. Here, we developed an artificial bioconversion process to synthesize L-phenylalanine from inexpensive aromatic precursors (benzaldehyde or benzyl alcohol). In particular, this work opens the possibility of L-phenylalanine production from benzyl alcohol in a cofactor self-sufficient system without any addition of reductant.
Results: The engineered L-phenylalanine biosynthesis pathway comprises two modules: in the first module, aromatic precursors and glycine were converted into phenylpyruvate, the key precursor for L-phenylalanine. The highly active enzyme combination was natural threonine aldolase LtaEP.p and threonine dehydratase A8HB.t, which could produce phenylpyruvate in a titer of 4.3 g/L. Overexpression of gene ridA could further increase phenylpyruvate production by 16.3%, reaching up to 5 g/L. The second module catalyzed phenylpyruvate to L-phenylalanine, and the conversion rate of phenylpyruvate was up to 93% by co-expressing PheDH and FDHV120S. Then, the engineered E. coli containing these two modules could produce L-phenylalanine from benzaldehyde with a conversion rate of 69%. Finally, we expanded the aromatic precursors to produce L-phenylalanine from benzyl alcohol, and firstly constructed the cofactor self-sufficient biosynthetic pathway to synthesize L-phenylalanine without any additional reductant such as formate.
Conclusion: Systematical bioconversion processes have been designed and constructed, which could provide a potential bio-based strategy for the production of high-value L-phenylalanine from low-cost starting materials aromatic precursors.
Keywords: Aromatic precursors; Benzaldehyde; Benzyl alcohol; Engineering; Escherichia coli; L-phenylalanine.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
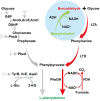
 indicate the repression and inhibition of the relevant genes in native pathway. Metabolites abbreviations: G6P glucose 6-phosphate, DAHP 3-deoxy-d-arabino- heptulosonate-7-phosphate, L-Glu L-glutamate, 2-KG 2-ketoglutarate
indicate the repression and inhibition of the relevant genes in native pathway. Metabolites abbreviations: G6P glucose 6-phosphate, DAHP 3-deoxy-d-arabino- heptulosonate-7-phosphate, L-Glu L-glutamate, 2-KG 2-ketoglutarate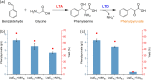




Similar articles
-
Shifting the biotransformation pathways of L-phenylalanine into benzaldehyde by Trametes suaveolens CBS 334.85 using HP20 resin.Lett Appl Microbiol. 2001 Apr;32(4):262-7. doi: 10.1046/j.1472-765x.2001.0873a.x. Lett Appl Microbiol. 2001. PMID: 11298938
-
One-Pot Cascade Biotransformation for Efficient Synthesis of Benzyl Alcohol and Its Analogs.Chem Asian J. 2020 Apr 1;15(7):1018-1021. doi: 10.1002/asia.201901680. Epub 2020 Feb 25. Chem Asian J. 2020. PMID: 32017396
-
Engineering Escherichia coli for renewable benzyl alcohol production.Metab Eng Commun. 2015 Jun 19;2:39-45. doi: 10.1016/j.meteno.2015.06.002. eCollection 2015 Dec. Metab Eng Commun. 2015. PMID: 34150507 Free PMC article.
-
Construction of recombinant Escherichia coli for production of L-phenylalanine-derived compounds.World J Microbiol Biotechnol. 2021 Apr 15;37(5):84. doi: 10.1007/s11274-021-03050-1. World J Microbiol Biotechnol. 2021. PMID: 33855641 Review.
-
Metabolic engineering for the production of l-phenylalanine in Escherichia coli.3 Biotech. 2019 Mar;9(3):85. doi: 10.1007/s13205-019-1619-6. Epub 2019 Feb 15. 3 Biotech. 2019. PMID: 30800596 Free PMC article. Review.
Cited by
-
Recent Advances in Metabolic Engineering for the Biosynthesis of Phosphoenol Pyruvate-Oxaloacetate-Pyruvate-Derived Amino Acids.Molecules. 2024 Jun 18;29(12):2893. doi: 10.3390/molecules29122893. Molecules. 2024. PMID: 38930958 Free PMC article. Review.
References
-
- Yuan P, Cao W, Wang Z, Chen K, Li Y, Ouyang P. Enhancement of L-phenylalanine production by engineered Escherichia coli using phased exponential L-tyrosine feeding combined with nitrogen source optimization. J Biosci Bioeng. 2015;120:36–40. - PubMed
-
- Hou Y, Hossain GS, Li J, Shin HD, Liu L, Du G. Production of phenylpyruvic acid from L-phenylalanine using an L-amino acid deaminase from Proteus mirabilis: comparison of enzymatic and whole-cell biotransformation approaches. Appl Microbiol Biotechnol. 2015;99:8391–8402. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

