Combination of mangiferin and T0901317 targeting autophagy promotes cholesterol efflux from macrophage foam cell in atherosclerosis
- PMID: 38183139
- PMCID: PMC10770909
- DOI: 10.1186/s13020-023-00876-9
Combination of mangiferin and T0901317 targeting autophagy promotes cholesterol efflux from macrophage foam cell in atherosclerosis
Abstract
Background: The synthetic liver X receptor ligand (LXR) T0901317 (T0) has been reported to attenuate atherosclerosis (AS) without hyperglyceridemia due to innovative drug combination or nano-sized drug delivery. Given the key roles of mangiferin (MGF) in lipid metabolism and atherogenesis, it is critical to investigate progression of atherosclerotic lesion after combined treatment of MGF and T0.
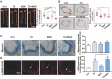
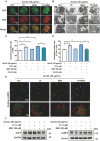
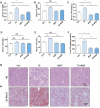
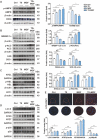
Methods: Atherosclerotic plaque formation and hepatic lipid accumulation were compared in Apoe-/- mice among T0 and/or MGF treatment. The in vitro functions of MGF and T0 were analyzed by Oil-red O staining, cholesterol efflux assay, transmission electron microscopy and western blot analyses with or without acetylated low density lipoprotein.
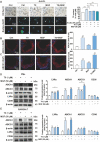
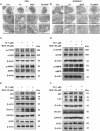
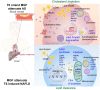
Results: The combination therapy are effective regulators for atherosclerotic plaque formation in Apoe-/- mice, due to upregulation of ABCA1 and ABCG1 induced by LXR activation. Subsequently, we identified autophagy promoted by MGF and T0 treatment establishes a positive feedback loop that increases cholesterol efflux, resulted from LXRα activation. Under atherogenic conditions, the autophagy inhibitor CQ abolished the enhancement effect on cholesterol outflow of MGF and T0. Mechanically, MGF and T0 promotes LXRα and mTOR/AMPK signaling cascade in macrophage, and promotes AMPK signaling cascade in hepatocyte, leading to lipid metabolic homeostasis.
Conclusions: Altogether, our findings reveal that MGF and T0 engages in AS therapy without side effects by activating AMPK-dependent autophagy to promote macrophage cholesterol efflux, and MGF might serve as a natural compound to assist T0 in AS via targeting autophagy.
Keywords: Autophagy; Cholesterol efflux; Macrophage foam cells; Mangiferin; T0901317.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Mangiferin promotes macrophage cholesterol efflux and protects against atherosclerosis by augmenting the expression of ABCA1 and ABCG1.Aging (Albany NY). 2019 Dec 2;11(23):10992-11009. doi: 10.18632/aging.102498. Epub 2019 Dec 2. Aging (Albany NY). 2019. PMID: 31790366 Free PMC article.
-
Leonurine Prevents Atherosclerosis Via Promoting the Expression of ABCA1 and ABCG1 in a Pparγ/Lxrα Signaling Pathway-Dependent Manner.Cell Physiol Biochem. 2017;43(4):1703-1717. doi: 10.1159/000484031. Epub 2017 Oct 18. Cell Physiol Biochem. 2017. PMID: 29045950
-
Homocysteine accelerates atherosclerosis via inhibiting LXRα-mediated ABCA1/ABCG1-dependent cholesterol efflux from macrophages.Life Sci. 2018 Dec 1;214:41-50. doi: 10.1016/j.lfs.2018.10.060. Epub 2018 Oct 28. Life Sci. 2018. PMID: 30393020
-
A novel therapeutic strategy for atherosclerosis: autophagy-dependent cholesterol efflux.J Physiol Biochem. 2022 Aug;78(3):557-572. doi: 10.1007/s13105-021-00870-5. Epub 2022 Jan 22. J Physiol Biochem. 2022. PMID: 35064467 Review.
-
Therapeutic potential of mangiferin in cancer: Unveiling regulatory pathways, mechanisms of action, and bioavailability enhancements - An updated review.Food Sci Nutr. 2023 Dec 20;12(3):1413-1429. doi: 10.1002/fsn3.3869. eCollection 2024 Mar. Food Sci Nutr. 2023. PMID: 38455223 Free PMC article. Review.
Cited by
-
Crosstalk between lipid metabolism and macrophages in atherosclerosis: therapeutic potential of natural products.Front Cardiovasc Med. 2025 Mar 3;12:1529924. doi: 10.3389/fcvm.2025.1529924. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40099271 Free PMC article. Review.
References
Grants and funding
- 82304809/National Natural Science Foundation of China
- GTZK202208/State Key Laboratory of Bioactive Substance and Function of Natural Medicines
- ZYYCXTD-C-202009/Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine
- 2021KJ133/Scientific Research Project of Tianjin Educational Committee
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

