Mechanical Models of Collagen Networks for Understanding Changes in the Failure Properties of Aging Skin
- PMID: 38183223
- PMCID: PMC10983714
- DOI: 10.1115/1.4064406
Mechanical Models of Collagen Networks for Understanding Changes in the Failure Properties of Aging Skin
Abstract
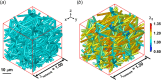
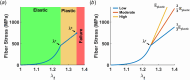
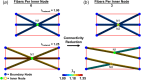
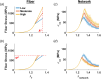
Skin undergoes mechanical alterations due to changes in the composition and structure of the collagenous dermis with aging. Previous studies have conflicting findings, with both increased and decreased stiffness reported for aging skin. The underlying structure-function relationships that drive age-related changes are complex and difficult to study individually. One potential contributor to these variations is the accumulation of nonenzymatic crosslinks within collagen fibers, which affect dermal collagen remodeling and mechanical properties. Specifically, these crosslinks make individual fibers stiffer in their plastic loading region and lead to increased fragmentation of the collagenous network. To better understand the influence of these changes, we investigated the impact of nonenzymatic crosslink changes on the dermal microstructure using discrete fiber networks representative of the dermal microstructure. Our findings suggest that stiffening the plastic region of collagen's mechanical response has minimal effects on network-level stiffness and failure stresses. Conversely, simulating fragmentation through a loss of connectivity substantially reduces network stiffness and failure stress, while increasing stretch ratios at failure.
Keywords: aging; biomechanics; collagen; dermis; fiber networks; multiphoton microscopy; non-affine; tissue damage.
Copyright © 2024 by ASME.
Figures


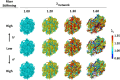
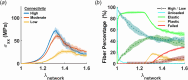

Similar articles
-
Prediction, screening and characterization of novel bioactive tetrapeptide matrikines for skin rejuvenation.Br J Dermatol. 2024 Jun 20;191(1):92-106. doi: 10.1093/bjd/ljae061. Br J Dermatol. 2024. PMID: 38375775
-
Quantitative polarization microscopy as a potential tool for quantification of mechanical stresses within 3D matrices.Acta Biomater. 2025 Jun 15;200:236-250. doi: 10.1016/j.actbio.2025.04.052. Epub 2025 May 9. Acta Biomater. 2025. PMID: 40348695
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
How lived experiences of illness trajectories, burdens of treatment, and social inequalities shape service user and caregiver participation in health and social care: a theory-informed qualitative evidence synthesis.Health Soc Care Deliv Res. 2025 Jun;13(24):1-120. doi: 10.3310/HGTQ8159. Health Soc Care Deliv Res. 2025. PMID: 40548558
Cited by
-
Data-driven continuum damage mechanics with built-in physics.Extreme Mech Lett. 2024 Sep;71:102220. doi: 10.1016/j.eml.2024.102220. Epub 2024 Aug 10. Extreme Mech Lett. 2024. PMID: 39372561
-
A multiscale discrete fiber model of failure in heterogeneous tissues: Applications to remodeled cerebral aneurysms.J Biomech. 2025 Jan;178:112343. doi: 10.1016/j.jbiomech.2024.112343. Epub 2024 Sep 24. J Biomech. 2025. PMID: 39341733
-
Quantification of age-related changes in the structure and mechanical function of skin with multiscale imaging.Geroscience. 2024 Oct;46(5):4869-4882. doi: 10.1007/s11357-024-01199-9. Epub 2024 May 18. Geroscience. 2024. PMID: 38761286 Free PMC article.
References
-
- Kanitakis, J. , 2002, “ Anatomy, Histology and Immunohistochemistry of Normal Human Skin,” Eur. J. Dermatol., 12(4), pp. 390–400.https://pubmed.ncbi.nlm.nih.gov/12095893/ - PubMed
-
- Sen, C. K. , Gordillo, G. M. , Roy, S. , Kirsner, R. , Lambert, L. , Hunt, T. K. , Gottrup, F. , Gurtner, G. C. , and Longaker, M. T. , 2009, “ Human Skin Wounds: A Major and Snowballing Threat to Public Health and the Economy,” Wound Repair Regener., 17(6), pp. 763–771. 10.1111/j.1524-475X.2009.00543.x - DOI - PMC - PubMed
-
- Albanna, M. Z. , and Holmes, J. H. , 2016, Skin Tissue Engineering and Regenerative Medicine, Elsevier/AP, Academic Press is an Imprint of Elsevier, London; San Diego, CA, p. 443.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

