Brain energy metabolism: A roadmap for future research
- PMID: 38183680
- PMCID: PMC11102343
- DOI: 10.1111/jnc.16032
Brain energy metabolism: A roadmap for future research
Abstract
Although we have learned much about how the brain fuels its functions over the last decades, there remains much still to discover in an organ that is so complex. This article lays out major gaps in our knowledge of interrelationships between brain metabolism and brain function, including biochemical, cellular, and subcellular aspects of functional metabolism and its imaging in adult brain, as well as during development, aging, and disease. The focus is on unknowns in metabolism of major brain substrates and associated transporters, the roles of insulin and of lipid droplets, the emerging role of metabolism in microglia, mysteries about the major brain cofactor and signaling molecule NAD+, as well as unsolved problems underlying brain metabolism in pathologies such as traumatic brain injury, epilepsy, and metabolic downregulation during hibernation. It describes our current level of understanding of these facets of brain energy metabolism as well as a roadmap for future research.
Keywords: GLUT4; acetate; aerobic glycolysis; insulin; noradrenaline.
© 2024 The Authors. Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
Caroline Rae is President of the International Society for Neurochemistry, the owner of this journal. Mary C. McKenna and João M.N. Duarte are Editors for the Journal of Neurochemistry. Kelly Drew has shown financial interest in Be Cool Pharmaceutics. Karin Borges declares consulting fees received from Ultragenyx Pharmaceuticals Inc. and Nestlé Purina PetCare in the past. Joseph Baur has received research funding, materials, and consulting fees from Pfizer, Cytokinetics, Elysium Health, and Metro International Biotech; he holds a patent for using NAD+ precursors in liver injury. Robert Zorec is Director and Founder of Celica Biomedical, a concessionaire for research funded by the government of R. Slovenia. The following state that they have no conflicts of interest: Gerald Dienel, Carlos Manlio Díaz-García, Kelly Drew, Timothy A Ryan, Douglas L Rothman, Michael B Robinson, Oliver Kann, Raymond A Swanson, Tibor Kristian, Dasfne Lee-Liu, Gökhan Uruk, Benjamin D Rowlands, Jordi Duran, Nina Vardjan, Britta Lindquist, William Shuttleworth, Ewan McNay, and Starlette Douglass.
Figures


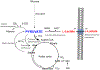

References
-
- Abate MG, Trivedi M, Fryer TD, Smielewski P, Chatfield DA, Williams GB, Aigbirhio F, Carpenter TA, Pickard JD, Menon DK, & Coles JP (2008). Early derangements in oxygen and glucose metabolism following head injury: The ischemic penumbra and pathophysiological heterogeneity. Neurocritical Care, 9, 319–325. - PubMed
-
- Adalbert R, & Coleman MP (2013). Review: Axon pathology in age-related neurodegenerative disorders. Neuropathology and Applied Neurobiology, 39, 90–108. - PubMed
Publication types
MeSH terms
Grants and funding
- I01 BX004895/BX/BLRD VA/United States
- R01 NS117139/NS/NINDS NIH HHS/United States
- P20 GM109089/GM/NIGMS NIH HHS/United States
- R01 NS070280/NS/NINDS NIH HHS/United States
- R01 NS106901/NS/NINDS NIH HHS/United States
- R01 NS110808/NS/NINDS NIH HHS/United States
- International Society for Cerebral Blood Flow and Metabolism
- R13 AG067693/AG/NIA NIH HHS/United States
- R01 NS106693/NS/NINDS NIH HHS/United States
- R37 NS036942/NS/NINDS NIH HHS/United States
- P01 HD085928/HD/NICHD NIH HHS/United States
- R01 AG066653/AG/NIA NIH HHS/United States
- ISN-CC Funding for a small conference/International Society for Neurochemistry
- R01 NS125653/NS/NINDS NIH HHS/United States
- R01 NS119275/NS/NINDS NIH HHS/United States
- R01 NS099461/NS/NINDS NIH HHS/United States
- P20 GM125528/GM/NIGMS NIH HHS/United States

