Genomic profiling in GIST: Implications in clinical outcome and future challenges
- PMID: 38183711
- PMCID: PMC10808967
- DOI: 10.1016/j.neo.2023.100959
Genomic profiling in GIST: Implications in clinical outcome and future challenges
Abstract
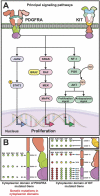
Gastrointestinal Stromal Tumors (GIST) are the most frequent mesenchymal neoplasia of the digestive tract. Genomic alterations in KIT, PDFGRA, SDH, and BRAF genes are essential in GIST oncogenesis. Therefore, the mutations in these genes have demonstrated clinical implications. Tumors with deletions in KIT-exon 11 or duplications in exon 9 are associated with a worse prognosis. In contrast, KIT-exon 11 substitutions and duplications are associated with a better clinical outcome. Moreover, mutations in Kit exon 9 and 11 are actionable, due to their response to imatinib, while mutations in PDGFRA respond to sunitinib and/or avapritinib. Although, molecular testing on tissue samples is effective; it is invasive, requires adequate amounts of tissue, and a long experimental process is needed for results. In contrast, liquid biopsy has been proposed as a simple and non-invasive method to test biomarkers in cancer. The most common molecule analyzed by liquid biopsy is circulating tumor DNA (ctDNA). GISTs ctDNA testing has been demonstrated to be effective in identifying known and novel KIT mutations that were not detected using traditional tissue DNA testing and have been useful in determining progression risk and response to TKI therapy. This allows the clinician to have an accurate picture of the genetic changes of the tumor over time. In this work, we aimed to discuss the implications of mutational testing in clinical outcomes, the methods to test ctDNA and the future challenges in the establishment of alternatives of personalized medicine.
Keywords: GIST; Genomic profiling; KIT; Mutations; PDGFRA; Treatment.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare no conflict of interest. This manuscript has not been submitted in other journal.
Figures

Similar articles
-
Circulating tumor DNA analysis of the phase III VOYAGER trial: KIT mutational landscape and outcomes in patients with advanced gastrointestinal stromal tumor treated with avapritinib or regorafenib.Ann Oncol. 2023 Jul;34(7):615-625. doi: 10.1016/j.annonc.2023.04.006. Epub 2023 Apr 25. Ann Oncol. 2023. PMID: 37105265 Free PMC article. Clinical Trial.
-
[The importance of mutational status in prognosis and therapy of GIST].Recenti Prog Med. 2015 Jan;106(1):17-22. doi: 10.1701/1740.18950. Recenti Prog Med. 2015. PMID: 25621775 Italian.
-
Effect of KIT and PDGFRA Mutations on Survival in Patients With Gastrointestinal Stromal Tumors Treated With Adjuvant Imatinib: An Exploratory Analysis of a Randomized Clinical Trial.JAMA Oncol. 2017 May 1;3(5):602-609. doi: 10.1001/jamaoncol.2016.5751. JAMA Oncol. 2017. PMID: 28334365 Free PMC article. Clinical Trial.
-
KIT and PDGFRA mutations in gastrointestinal stromal tumors (GISTs).Semin Diagn Pathol. 2006 May;23(2):91-102. doi: 10.1053/j.semdp.2006.08.006. Semin Diagn Pathol. 2006. PMID: 17193822 Review.
-
[Translational research and diagnosis in GIST].Pathologe. 2012 Nov;33 Suppl 2:273-7. doi: 10.1007/s00292-012-1682-9. Pathologe. 2012. PMID: 22968735 Review. German.
Cited by
-
Prognostic Significance of Circulating Tumor DNA Mutations in Gastrointestinal Stromal Tumors: A Systematic Review and Meta-analysis Based on Time-To-Event Data.J Gastrointest Cancer. 2025 Jul 15;56(1):153. doi: 10.1007/s12029-025-01271-3. J Gastrointest Cancer. 2025. PMID: 40665034
-
Potential biomarkers for the prognosis of gastrointestinal stromal tumors.World J Gastrointest Oncol. 2025 Apr 15;17(4):102831. doi: 10.4251/wjgo.v17.i4.102831. World J Gastrointest Oncol. 2025. PMID: 40235893 Free PMC article.
-
Trends and outcomes for patients receiving neoadjuvant therapy for stage I to III gastric gastrointestinal stromal tumors.J Gastrointest Surg. 2025 Aug;29(8):102117. doi: 10.1016/j.gassur.2025.102117. Epub 2025 Jun 16. J Gastrointest Surg. 2025. PMID: 40516690
-
Exploring nanotechnology solutions for improved outcomes in gastrointestinal stromal tumors.Heliyon. 2024 Nov 22;10(23):e40596. doi: 10.1016/j.heliyon.2024.e40596. eCollection 2024 Dec 15. Heliyon. 2024. PMID: 39687122 Free PMC article. Review.
-
BUB1 an Overexpressed Kinase in Sarcoma: Finding New Target Therapy for Osteosarcoma, Liposarcoma, Synovial Sarcoma, and Leiomyosarcoma.Biomolecules. 2025 Jul 18;15(7):1046. doi: 10.3390/biom15071046. Biomolecules. 2025. PMID: 40723917 Free PMC article.
References
-
- Mandahl, N.; Mertens, F. Soft tissue tumors; 2010; ISBN 9780470181799.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

