Chloroplasts lacking class I glutaredoxins are functional but show a delayed recovery of protein cysteinyl redox state after oxidative challenge
- PMID: 38183796
- PMCID: PMC10808970
- DOI: 10.1016/j.redox.2023.103015
Chloroplasts lacking class I glutaredoxins are functional but show a delayed recovery of protein cysteinyl redox state after oxidative challenge
Abstract
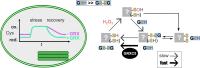
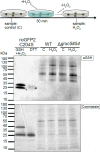
Redox status of protein cysteinyl residues is mediated via glutathione (GSH)/glutaredoxin (GRX) and thioredoxin (TRX)-dependent redox cascades. An oxidative challenge can induce post-translational protein modifications on thiols, such as protein S-glutathionylation. Class I GRX are small thiol-disulfide oxidoreductases that reversibly catalyse S-glutathionylation and protein disulfide formation. TRX and GSH/GRX redox systems can provide partial backup for each other in several subcellular compartments, but not in the plastid stroma where TRX/light-dependent redox regulation of primary metabolism takes place. While the stromal TRX system has been studied at detail, the role of class I GRX on plastid redox processes is still unknown. We generate knockout lines of GRXC5 as the only chloroplast class I GRX of the moss Physcomitrium patens. While we find that PpGRXC5 has high activities in GSH-dependent oxidoreductase assays using hydroxyethyl disulfide or redox-sensitive GFP2 as substrates in vitro, Δgrxc5 plants show no detectable growth defect or stress sensitivity, in contrast to mutants with a less negative stromal EGSH (Δgr1). Using stroma-targeted roGFP2, we show increased protein Cys steady state oxidation and decreased reduction rates after oxidative challenge in Δgrxc5 plants in vivo, indicating kinetic uncoupling of the protein Cys redox state from EGSH. Compared to wildtype, protein Cys disulfide formation rates and S-glutathionylation levels after H2O2 treatment remained unchanged. Lack of class I GRX function in the stroma did not result in impaired carbon fixation. Our observations suggest specific roles for GRXC5 in the efficient transfer of electrons from GSH to target protein Cys as well as negligible cross-talk with metabolic regulation via the TRX system. We propose a model for stromal class I GRX function in efficient catalysis of protein dithiol/disulfide equilibria upon redox steady state alterations affecting stromal EGSH and highlight the importance of identifying in vivo target proteins of GRXC5.
Keywords: E(GSH); GRXC5; Genetically encoded biosensor; Glutaredoxin; Glutathione; Photosynthesis; Plastid; Redox-sensitive GFP; S-glutathionylation.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures







Similar articles
-
Reduction potentials of protein disulfides and catalysis of glutathionylation and deglutathionylation by glutaredoxin enzymes.Biochem J. 2017 Nov 9;474(22):3799-3815. doi: 10.1042/BCJ20170589. Biochem J. 2017. PMID: 28963348
-
One cysteine is enough: A monothiol Grx can functionally replace all cytosolic Trx and dithiol Grx.Redox Biol. 2020 Sep;36:101598. doi: 10.1016/j.redox.2020.101598. Epub 2020 May 31. Redox Biol. 2020. PMID: 32521506 Free PMC article.
-
Glutaredoxin accelerates glutathione-dependent folding of reduced ribonuclease A together with protein disulfide-isomerase.J Biol Chem. 1995 Apr 7;270(14):7822-8. doi: 10.1074/jbc.270.14.7822. J Biol Chem. 1995. PMID: 7713872
-
Glutathione and Glutaredoxin-Key Players in Cellular Redox Homeostasis and Signaling.Antioxidants (Basel). 2023 Aug 3;12(8):1553. doi: 10.3390/antiox12081553. Antioxidants (Basel). 2023. PMID: 37627548 Free PMC article. Review.
-
The emerging roles of protein glutathionylation in chloroplasts.Plant Sci. 2012 Apr;185-186:86-96. doi: 10.1016/j.plantsci.2012.01.005. Epub 2012 Jan 23. Plant Sci. 2012. PMID: 22325869 Review.
Cited by
-
Genome-wide identification and expression analysis of glutaredoxin in Puccinellia tenuiflora under salinity stress.BMC Plant Biol. 2025 May 8;25(1):605. doi: 10.1186/s12870-025-06547-1. BMC Plant Biol. 2025. PMID: 40340753 Free PMC article.
-
Turning the knobs: The impact of glutathionylation on starch metabolism.Plant Physiol. 2025 Aug 4;198(4):kiaf322. doi: 10.1093/plphys/kiaf322. Plant Physiol. 2025. PMID: 40700547 Free PMC article. No abstract available.
-
Dynamic regulation of Arabidopsis β-AMYLASE1 by glutathione and thioredoxins affects starch in guard cells.Plant Physiol. 2025 Aug 4;198(4):kiaf344. doi: 10.1093/plphys/kiaf344. Plant Physiol. 2025. PMID: 40749095 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

