Elucidating cellular response to treatment with viral immunotherapies in pediatric high-grade glioma and medulloblastoma
- PMID: 38183802
- PMCID: PMC10809117
- DOI: 10.1016/j.tranon.2024.101875
Elucidating cellular response to treatment with viral immunotherapies in pediatric high-grade glioma and medulloblastoma
Abstract
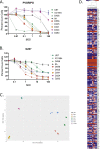
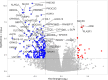
HSV G207, a double-stranded, DNA virus, and the polio:rhinovirus chimera, PVSRIPO, a single positive-strand RNA virus, are viral immunotherapies being used to treat pediatric malignant brain tumors in clinical trials. The purpose of this work is to elucidate general response patterns and putative biomarkers of response. Multiple pediatric high-grade glioma and medulloblastoma cell lines were treated with various multiplicities of infection of G207 or PVSRIPO. There was a significant inverse correlation between expression of one HSV cellular receptor, CD111, and the lethal dose of 50% of cells (LD50) of cells treated with G207 (r = -0.985, P<0.001) but no correlation between PVSRIPO cellular receptor expression (CD155) and LD50. RNA sequencing of control cells and cells treated for 8 and 24 h revealed that there were few shared differentially expressed (DE) genes between cells treated with PVSRIPO and G207: GCLM, LANCL2, and RBM3 were enriched whilst ADAMTS1 and VEGFA were depleted. Likewise, there were few shared DE genes enriched between medulloblastoma and high-grade glioma cell lines treated with G207: GPSM2, CHECK2, SEPTIN2, EIF4G2, GCLM, GDAP1, LANCL2, and PWP1. Treatment with G207 and PVSRIPO appear to cause disparate gene enrichment and depletion suggesting disparate molecular mechanisms in malignant pediatric brain tumors.
Keywords: Cell response; G207; Medulloblastoma; Oncolytic virus; PVSRIPO; Pediatric high-grade glioma; RNA sequencing; Viral immunotherapy.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest Two authors, Michael Brown and Matthias Gromeier own intellectual property related to PVSRIPO, which has been licensed to Istari Oncology, Inc. Matthias Gromeier holds equity in Istari Oncology, Inc. Drs. Brown and Gromeier received consultancy fees from Istari Oncology, Inc.
Figures




Similar articles
-
Poliovirus Receptor (CD155) Expression in Pediatric Brain Tumors Mediates Oncolysis of Medulloblastoma and Pleomorphic Xanthoastrocytoma.J Neuropathol Exp Neurol. 2018 Aug 1;77(8):696-702. doi: 10.1093/jnen/nly045. J Neuropathol Exp Neurol. 2018. PMID: 29878245 Free PMC article.
-
Oncolytic HSV-1 G207 Immunovirotherapy for Pediatric High-Grade Gliomas.N Engl J Med. 2021 Apr 29;384(17):1613-1622. doi: 10.1056/NEJMoa2024947. Epub 2021 Apr 10. N Engl J Med. 2021. PMID: 33838625 Free PMC article.
-
CD155 is a putative therapeutic target in medulloblastoma.Clin Transl Oncol. 2023 Mar;25(3):696-705. doi: 10.1007/s12094-022-02975-9. Epub 2022 Oct 27. Clin Transl Oncol. 2023. PMID: 36301489
-
Clinical advances in oncolytic virotherapy for pediatric brain tumors.Pharmacol Ther. 2022 Nov;239:108193. doi: 10.1016/j.pharmthera.2022.108193. Epub 2022 Apr 26. Pharmacol Ther. 2022. PMID: 35487285 Free PMC article. Review.
-
Oncolytic virotherapies for pediatric tumors.Expert Opin Biol Ther. 2023 Jul-Dec;23(10):987-1003. doi: 10.1080/14712598.2023.2245326. Epub 2023 Oct 5. Expert Opin Biol Ther. 2023. PMID: 37749907 Free PMC article. Review.
Cited by
-
Oncolytic viruses: a promising therapy for malignant pleural effusion and solid tumors.Front Immunol. 2025 Apr 25;16:1570698. doi: 10.3389/fimmu.2025.1570698. eCollection 2025. Front Immunol. 2025. PMID: 40352942 Free PMC article. Review.
-
Direct oHSV Infection Induces DC Maturation and a Tumor Therapeutic Response.Viruses. 2025 Aug 19;17(8):1134. doi: 10.3390/v17081134. Viruses. 2025. PMID: 40872847 Free PMC article.
-
Epigenetic regulation of the human GDAP1 gene.Biochem Biophys Rep. 2024 Sep 19;40:101827. doi: 10.1016/j.bbrep.2024.101827. eCollection 2024 Dec. Biochem Biophys Rep. 2024. PMID: 39328838 Free PMC article.
References
-
- Thompson E.M., Hielscher T., Bouffet E., Remke M., Luu B., Gururangan S., McLendon R.E., Bigner D.D., Lipp E.S., Perreault S., Cho Y.J., et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol. 2016;17:484–495. doi: 10.1016/S1470-2045(15)00581-1. - DOI - PMC - PubMed
-
- Jakacki R.I., Cohen K.J., Buxton A., Krailo M.D., Burger P.C., Rosenblum M.K., Brat D.J., Hamilton R.L., Eckel S.P., Zhou T., Lavey R.S., et al. Phase 2 study of concurrent radiotherapy and temozolomide followed by temozolomide and lomustine in the treatment of children with high-grade glioma: a report of the children's oncology group ACNS0423 study. Neuro. Oncol. 2016;18:1442–1450. doi: 10.1093/neuonc/now038. - DOI - PMC - PubMed
-
- Thompson E.M., Landi D., Brown M.C., Friedman H.S., McLendon R., Herndon J.E., 2nd, Buckley E., Bolognesi D.P., Lipp E., Schroeder K., Becher O.J., et al. Recombinant polio-rhinovirus immunotherapy for recurrent paediatric high-grade glioma: a phase 1b trial. Lancet Child Adolesc. Health. 2023 doi: 10.1016/S2352-4642(23)00031-7. - DOI - PMC - PubMed
-
- Desjardins A., Gromeier M., Herndon J.E., 2nd, Beaubier N., Bolognesi D.P., Friedman A.H., Friedman H.S., McSherry F., Muscat A.M., Nair S., Peters K.B., et al. Recurrent glioblastoma treated with recombinant poliovirus. N. Engl. J. Med. 2018;379:150–161. doi: 10.1056/NEJMoa1716435. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

