Blood DNA methylation profiling identifies cathepsin Z dysregulation in pulmonary arterial hypertension
- PMID: 38184627
- PMCID: PMC10771427
- DOI: 10.1038/s41467-023-44683-0
Blood DNA methylation profiling identifies cathepsin Z dysregulation in pulmonary arterial hypertension
Abstract
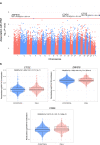
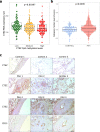
Pulmonary arterial hypertension (PAH) is characterised by pulmonary vascular remodelling causing premature death from right heart failure. Established DNA variants influence PAH risk, but susceptibility from epigenetic changes is unknown. We addressed this through epigenome-wide association study (EWAS), testing 865,848 CpG sites for association with PAH in 429 individuals with PAH and 1226 controls. Three loci, at Cathepsin Z (CTSZ, cg04917472), Conserved oligomeric Golgi complex 6 (COG6, cg27396197), and Zinc Finger Protein 678 (ZNF678, cg03144189), reached epigenome-wide significance (p < 10-7) and are hypermethylated in PAH, including in individuals with PAH at 1-year follow-up. Of 16 established PAH genes, only cg10976975 in BMP10 shows hypermethylation in PAH. Hypermethylation at CTSZ is associated with decreased blood cathepsin Z mRNA levels. Knockdown of CTSZ expression in human pulmonary artery endothelial cells increases caspase-3/7 activity (p < 10-4). DNA methylation profiles are altered in PAH, exemplified by the pulmonary endothelial function modifier CTSZ, encoding protease cathepsin Z.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

