Dynamic changes in mitral valve extracellular matrix, tissue mechanics and function in a mouse model of Marfan syndrome
- PMID: 38185344
- PMCID: PMC12034306
- DOI: 10.1016/j.matbio.2024.01.001
Dynamic changes in mitral valve extracellular matrix, tissue mechanics and function in a mouse model of Marfan syndrome
Abstract
Objective: Mouse models of Marfan syndrome (MFS) with Fibrillin 1 (Fbn1) variant C1041G exhibit cardiovascular abnormalities, including myxomatous valve disease (MVD) and aortic aneurism, with structural extracellular matrix (ECM) dysregulation. In this study, we examine the structure-function-mechanics relations of the mitral valve related to specific transitions in ECM composition and organization in progressive MVD in MFS mice from Postnatal day (P)7 to 1 year-of-age.
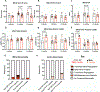
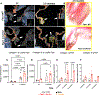
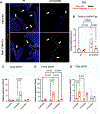
Approach and results: Mechanistic links between mechanical forces and biological changes in MVD progression were examined in Fbn1C1041G/+ MFS mice. By echocardiography, mitral valve dysfunction is prevalent at 2 months with a decrease in cardiac function at 6 months, followed by a preserved cardiac function at 12 months. Mitral valve (MV) regurgitation occurs in a subset of mice at 2-6 months, while progressive dilatation of the aorta occurs from 2 to 12 months. Mitral valve tissue mechanical assessments using a uniaxial Permeabilizable Fiber System demonstrate decreased stiffness of MFS MVs at all stages. Histological and microscopic analysis of ECM content, structure, and fiber orientation demonstrate that alterations in ECM mechanics, composition, and organization precede functional abnormalities in Fbn1C1041G/+MFS MVs. At 2 months, ECM abnormalities are detected with an increase in proteoglycans and decreased stiffness of the mitral valve. By 6-12 months, collagen fiber remodeling is increased with abnormal fiber organization in MFS mitral valve leaflets. At the same time, matrifibrocyte gene expression characteristic of collagen-rich connective tissue is increased, as detected by RNA in situ hybridization and qPCR. Together, these studies demonstrate early prevalence of proteoglycans at 2 months followed by upregulation of collagen structure and organization with age in MVs of MFS mice.
Conclusions: Altogether, our data indicate dynamic regulation of mitral valve structure, tissue mechanics, and function that reflect changes in ECM composition, organization, and gene expression in progressive MVD. Notably, increased collagen fiber organization and orientation, potentially dependent on increased matrifibrocyte cell activity, is apparent with altered mitral valve mechanics and function in aging MFS mice.
Keywords: Collagen organization and structure; Myxomatous mitral valve; Proteoglycans; Valve function; Valve tissue stiffness.
Copyright © 2024. Published by Elsevier B.V.
Figures





Similar articles
-
Deficiency of Circulating Monocytes Ameliorates the Progression of Myxomatous Valve Degeneration in Marfan Syndrome.Circulation. 2020 Jan 14;141(2):132-146. doi: 10.1161/CIRCULATIONAHA.119.042391. Epub 2020 Jan 13. Circulation. 2020. PMID: 31928435 Free PMC article.
-
Wnt Signaling Inhibition Prevents Postnatal Inflammation and Disease Progression in Mouse Congenital Myxomatous Valve Disease.Arterioscler Thromb Vasc Biol. 2024 Jul;44(7):1540-1554. doi: 10.1161/ATVBAHA.123.320388. Epub 2024 Apr 25. Arterioscler Thromb Vasc Biol. 2024. PMID: 38660802 Free PMC article.
-
Loss of Axin2 results in impaired heart valve maturation and subsequent myxomatous valve disease.Cardiovasc Res. 2017 Jan;113(1):40-51. doi: 10.1093/cvr/cvw229. Epub 2016 Nov 7. Cardiovasc Res. 2017. PMID: 28069701 Free PMC article.
-
Cardiovascular characteristics in Marfan syndrome and their relation to the genotype.Verh K Acad Geneeskd Belg. 2009;71(6):335-71. Verh K Acad Geneeskd Belg. 2009. PMID: 20232788 Review.
-
Mitral valve disease in Marfan syndrome and related disorders.J Cardiovasc Transl Res. 2011 Dec;4(6):741-7. doi: 10.1007/s12265-011-9314-y. Epub 2011 Aug 25. J Cardiovasc Transl Res. 2011. PMID: 21866385 Review.
Cited by
-
Multidimensional excavation of the current status and trends of mechanobiology in cardiovascular homeostasis and remodeling within 20 years.Mechanobiol Med. 2025 Mar 19;3(2):100127. doi: 10.1016/j.mbm.2025.100127. eCollection 2025 Jun. Mechanobiol Med. 2025. PMID: 40395770 Free PMC article. Review.
References
-
- Hinton RB Jr., et al., Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res, 2006. 98(11): p. 1431–8. - PubMed
-
- Nishimura RA, Vahanian A, Eleid MF, and Mack MJ, Mitral valve disease--current management and future challenges. Lancet, 2016. 387(10025): p. 1324–34. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

