[Impact of SARS-CoV-2 infection on graft composition and early transplant outcomes following allogeneic hematopoietic stem cell transplantation]
- PMID: 38185517
- PMCID: PMC10753252
- DOI: 10.3760/cma.j.issn.0253-2727.2023.11.002
[Impact of SARS-CoV-2 infection on graft composition and early transplant outcomes following allogeneic hematopoietic stem cell transplantation]
Abstract
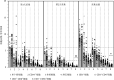
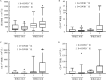
Objective: To assess the feasibility of using donors with novel coronavirus disease 2019 (COVID-19) for allogeneic hematopoietic stem cell transplantation (allo-HSCT) when there are no other available donors and allo-HSCT cannot be delayed or discontinued. Methods: Seventy-one patients with malignant hematological diseases undergoing allo-HSCT between December 8, 2022, and January 10, 2023, were included. Of these, 16 received grafts from donors with mild COVID-19 (D-COVID(+) group) and 55 received grafts from donors without COVID-19 (D-COVID(-) group). The graft compositions were compared between the two groups. Engraftment, acute graft-versus-host disease (aGVHD), overall survival (OS), and relapse were also evaluated. Results: There were no serious side effects or adverse events in the D-COVID(+) group. The mononuclear cell dose and CD34(+) cell dose were comparable between the two groups, and no additional apheresis was required. There were no significant differences in the lymphocyte, monocyte, and T-cell subset doses between the two groups. The median natural killer cell dose in the D-COVID(+) group was significantly higher than that in the D-COVID(-) group (0.69×10(8)/kg vs. 0.53×10(8)/kg, P=0.031). The median follow-up time was 72 (33-104) days. All patients achieved primary engraftment. The 60-day platelet engraftment rates in the D-COVID(+) and D-COVID(-) groups were 100% and (96.4±0.2) %, respectively (P=0.568). There were no significant differences in neutrophil (P=0.309) and platelet (P=0.544) engraftment times. The cumulative incidence of grade 2-4 aGVHD was (37.5±1.6) % vs. (16.4±0.3) % (P=0.062), and of grade 3-4 aGVHD was 25.0% ±1.3% vs. 9.1% ±0.2% (P=0.095) in the D-COVID(+) and D-COVID(-) groups, respectively. The probabilities of 60-day OS were 100% and 98.1% ±1.8% (P=0.522) in the D-COVID(+) and D-COVID(-) groups, respectively. There was no relapse of primary disease during the study period. Conclusion: When allo-HSCT cannot be delayed or discontinued and no other donor is available, a donor with mild COVID-19 should be considered if tolerable. Larger sample sizes and longer follow-up periods are required to validate these results.
目的: 评估供者发生新型冠状病毒感染(COVID-19)时,在没有其他可用供者且异基因造血干细胞移植(allo-HSCT)无法推迟或中止的情况下,使用原计划供者进行allo-HSCT的可行性。 方法: 纳入71例在2022年12月8日至2023年1月10日接受allo-HSCT的恶性血液病患者,其中16例接受轻症新冠病毒感染供者移植物(D-COVID(+)组),55例接受未感染新冠病毒感染供者移植物(D-COVID(-)组)。比较D-COVID(+)组和D-COVID(-)组供者采集物组分,观察两组早期植入、急性移植物抗宿主病(aGVHD)、总生存和复发等结局。 结果: D-COVID(+)组供者未发生严重不良事件。与D-COVID(-)组相比,D-COVID(+)组移植物的单个核细胞(MNC)和CD34(+)细胞计数相当,且无需更多的采集次数。两组淋巴细胞、单核细胞、T细胞亚群分布差异无统计学意义,而D-COVID(+)组移植物中位NK细胞含量高于D-COVID(-)组(0.69×10(8)/kg对0.53×10(8)/kg,P=0.031)。中位随访时间为72(33~104)d。所有患者均获得粒细胞植入。D-COVID(+)组、D-COVID(-)组移植后60 d血小板植入率分别为100%、(96.4±0.2)%(P=0.568),并且粒细胞植入、血小板植入时间差异均无统计学意义(P=0.309,P=0.544)。D-COVID(+)组、D-COVID(-)组Ⅱ~Ⅳ度aGVHD累积发生率分别为(37.5±1.6)%、(16.4±0.3)%(P=0.062),Ⅲ/Ⅳ度aGVHD累积发生率分别为(25.0±1.3)%、(9.1±0.2)%(P=0.095),移植后60 d总生存率分别为100%、(98.1 ± 1.8)%(P=0.522)。随访期间未发生原发病复发。 结论: 在无替代供者且allo-HSCT无法推迟或中止时,如供者可耐受,可考虑使用轻型新型冠状病毒感染供者按原计划进行移植。.
Keywords: Allogeneic hematopoietic stem cell transplantation; COVID-19; Donor; Malignant hematological disease.
Conflict of interest statement
Figures
Similar articles
-
Recent infection with SARS-CoV-2 in donors was associated with a higher incidence of acute graft-versus-host disease in recipients undergoing allogeneic haematopoietic stem cell transplantation.Br J Haematol. 2024 Aug;205(2):452-462. doi: 10.1111/bjh.19594. Epub 2024 Jun 24. Br J Haematol. 2024. PMID: 38924065
-
Universal Engraftment after Allogeneic Hematopoietic Cell Transplantation Using Cryopreserved CD34-Selected Grafts.Transplant Cell Ther. 2021 Aug;27(8):697.e1-697.e5. doi: 10.1016/j.jtct.2021.04.026. Epub 2021 May 13. Transplant Cell Ther. 2021. PMID: 33991721 Free PMC article.
-
[Second allogeneic hematopoietic stem cell transplantation with reduced-intensity conditioning and donor changes in relapsed hematological malignancies after the first allogeneic transplant].Zhonghua Xue Ye Xue Za Zhi. 2023 Jun 14;44(6):465-471. doi: 10.3760/cma.j.issn.0253-2727.2023.06.004. Zhonghua Xue Ye Xue Za Zhi. 2023. PMID: 37550201 Free PMC article. Chinese.
-
Efficacy and safety of mesenchymal stem cells co-infusion in allogeneic hematopoietic stem cell transplantation: a systematic review and meta-analysis.Stem Cell Res Ther. 2021 Apr 20;12(1):246. doi: 10.1186/s13287-021-02304-x. Stem Cell Res Ther. 2021. PMID: 33879242 Free PMC article.
-
Outcomes with CD34-Selected Stem Cell Boost for Poor Graft Function after Allogeneic Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis.Transplant Cell Ther. 2021 Oct;27(10):877.e1-877.e8. doi: 10.1016/j.jtct.2021.07.012. Epub 2021 Jul 18. Transplant Cell Ther. 2021. PMID: 34284148
Cited by
-
The impact of the donors' COVID-19 status on the outcomes of allogeneic hematopoietic stem cell transplantation: a multi-center retrospective study.Front Microbiol. 2024 Jul 15;15:1415289. doi: 10.3389/fmicb.2024.1415289. eCollection 2024. Front Microbiol. 2024. PMID: 39077735 Free PMC article.
-
[Allogeneic hematopoietic stem cell transplantation during the normalization stage of COVID-19 management].Zhonghua Xue Ye Xue Za Zhi. 2024 Nov 14;45(11):977-981. doi: 10.3760/cma.j.cn121090-20240817-00308. Zhonghua Xue Ye Xue Za Zhi. 2024. PMID: 39746688 Free PMC article. Chinese.
References
-
- Zhang XH, Chen J, Han MZ, et al. The consensus from The Chinese Society of Hematology on indications, conditioning regimens and donor selection for allogeneic hematopoietic stem cell transplantation: 2021 update[J] J Hematol Oncol. 2021;14(1):145. doi: 10.1186/s13045-021-01159-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous