High-throughput detection of parasites and ova in stool using the fully automatic digital feces analyzer, orienter model fa280
- PMID: 38185634
- PMCID: PMC10771706
- DOI: 10.1186/s13071-023-06108-1
High-throughput detection of parasites and ova in stool using the fully automatic digital feces analyzer, orienter model fa280
Abstract
Background: Intestinal parasitic infections can harm health by causing malnutrition, anemia, impaired growth and cognitive development, and alterations in microbiota composition and immune responses. Therefore, it is crucial to examine stool samples to diagnose parasitic infections. However, the traditional microscopic detection method is time-consuming, labor-intensive, and dependent on the expertise and training of microscopists. Hence, there is a need for a low-complexity, high-throughput, and cost-effective alternative to labor-intensive microscopic examinations.
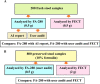
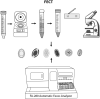
Methods: This study aimed to compare the performance of a fully automatic digital feces analyzer, Orienter Model FA280 (People's Republic of China) with that of the formalin-ethyl acetate concentration technique (FECT). We assessed and compared the agreement between the FA280 and the FECT for parasite detection and species identification in stool samples. The first part of the study analyzed 200 fresh stool samples for parasite detection using the FECT and FA280. With the FA280, the automatic feces analyzer performed the testing, and the digital microscope images were uploaded and automatically evaluated using an artificial intelligence (AI) program. Additionally, a skilled medical technologist conducted a user audit of the FA280 findings. The second set of samples comprised 800 preserved stool samples (preserved in 10% formalin). These samples were examined for parasites using the FECT and FA280 with a user audit.
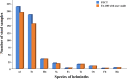
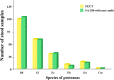
Results: For the first set of stool samples, there was no statistically significant difference in the pairwise agreements between the FECT and the FA280 with a user audit (exact binomial test, P = 1). However, there were statistically significant differences between the pairwise agreements for the FECT and the FA280 with the AI report (McNemar's test, P < 0.001). The agreement for the species identification of parasites between the FA280 with AI report and FECT showed fair agreement (overall agreement = 75.5%, kappa [κ] = 0.367, 95% CI 0.248-0.486). On the other hand, the user audit for the FA280 and FECT showed perfect agreement (overall agreement = 100%, κ = 1.00, 95% CI 1.00-1.00). For the second set of samples, the FECT detected significantly more positive samples for parasites than the FA280 with a user audit (McNemar's test, P < 0.001). The disparity in results may be attributed to the FECT using significantly larger stool samples than those used by the FA280. The larger sample size used by the FECT potentially contributed to the higher parasite detection rate. Regarding species identification, there was strong agreement between the FECT and the FA280 with a user audit for helminths (κ = 0.857, 95% CI 0.82-0.894). Similarly, there was perfect agreement for the species identification of protozoa between the FECT and the FA280 with user audit (κ = 1.00, 95% CI 1.00-1.00).
Conclusions: Although the FA280 has advantages in terms of simplicity, shorter performance time, and reduced contamination in the laboratory, there are some limitations to consider. These include a higher cost per sample testing and a lower sensitivity compared to the FECT. However, the FA280 enables rapid, convenient, and safe stool examination of parasitic infections.
Keywords: FA280 autoanalyzer; FECT; Formalin-ethyl acetate; Helminth; Parasitic detection; Protozoa.
© 2024. The Author(s).
Conflict of interest statement
This study received support from Firmer Co. Ltd., who provided the Orienter Model FA280 digital feces analyzer for our investigation. However, neither the personnel nor any affiliated entities of Firmer Co. Ltd. were involved in the conduct of this study. The authors declare that there are no competing interests in relation to this research.
Figures


Similar articles
-
Assessment of the application of the FA280-a fully automated fecal analyzer for diagnosing clonorchiasis: a mixed-method study.Infect Dis Poverty. 2025 Jan 6;14(1):1. doi: 10.1186/s40249-024-01271-8. Infect Dis Poverty. 2025. PMID: 39757228 Free PMC article.
-
Repeated stool sampling and use of multiple techniques enhance the sensitivity of helminth diagnosis: a cross-sectional survey in southern Lao People's Democratic Republic.Acta Trop. 2015 Jan;141(Pt B):315-21. doi: 10.1016/j.actatropica.2014.09.004. Epub 2014 Sep 16. Acta Trop. 2015. PMID: 25225157
-
Formalin-ethyl acetate concentration, FLOTAC Pellet and anal swab techniques for the diagnosis of intestinal parasites.Parasitol Res. 2018 Nov;117(11):3567-3573. doi: 10.1007/s00436-018-6054-9. Epub 2018 Aug 18. Parasitol Res. 2018. PMID: 30121754
-
Parasites in Human Stool: To Ignore or Not To Ignore?Pediatr Infect Dis J. 2019 Jun;38(6S Suppl 1):S47-S51. doi: 10.1097/INF.0000000000002323. Pediatr Infect Dis J. 2019. PMID: 31205245 Review.
-
Comparative studies on faecal egg counting techniques used for the detection of gastrointestinal parasites of equines: A systematic review.Curr Res Parasitol Vector Borne Dis. 2021 Aug 9;1:100046. doi: 10.1016/j.crpvbd.2021.100046. eCollection 2021. Curr Res Parasitol Vector Borne Dis. 2021. PMID: 35284858 Free PMC article. Review.
Cited by
-
Case Report: Endoscopic examination improves the diagnosis of inconspicuous helminth infections in adults in Shanghai.Front Med (Lausanne). 2025 May 21;12:1541099. doi: 10.3389/fmed.2025.1541099. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40470057 Free PMC article.
-
Laboratory validation of the automated diagnosis of intestinal parasites via fecal sample processing for the recovery of intestinal parasites through the dissolved air flotation technique.Parasit Vectors. 2024 Aug 30;17(1):368. doi: 10.1186/s13071-024-06434-y. Parasit Vectors. 2024. PMID: 39215369 Free PMC article.
-
Assessment of the application of the FA280-a fully automated fecal analyzer for diagnosing clonorchiasis: a mixed-method study.Infect Dis Poverty. 2025 Jan 6;14(1):1. doi: 10.1186/s40249-024-01271-8. Infect Dis Poverty. 2025. PMID: 39757228 Free PMC article.
-
Evaluation of the AiDx Assist device for automated detection of Schistosoma eggs in stool and urine samples in Nigeria.Front Parasitol. 2025 Mar 17;4:1440299. doi: 10.3389/fpara.2025.1440299. eCollection 2025. Front Parasitol. 2025. PMID: 40166406 Free PMC article.
-
The clinical application value of the KU-F40 fully automatic fecal analyzer for the detection of fecal parasites: A large-sample retrospective study.Sci Rep. 2025 Jul 7;15(1):24172. doi: 10.1038/s41598-025-09935-7. Sci Rep. 2025. PMID: 40624366 Free PMC article.
References
-
- Wattano S, Kerdpunya K, Keawphanuk P, Hunnangkul S, Loimak S, Tungtrongchitra A, et al. An epidemiological survey of intestinal parasitic infection and the socioeconomic status of the ethnic minority people of Moken and orang Laut. Trop Med Infect Dis. 2023;8:161–215. doi: 10.3390/tropicalmed8030161. - DOI - PMC - PubMed
-
- Sayasone S, Utzinger J, Akkhavong K, Odermatt P. Multiparasitism and intensity of helminth infections in relation to symptoms and nutritional status among children: a cross-sectional study in southern Lao People’s Democratic Republic. Acta Trop. 2015;141:322–331. doi: 10.1016/j.actatropica.2014.09.015. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

