Ischemia-reperfusion injury: molecular mechanisms and therapeutic targets
- PMID: 38185705
- PMCID: PMC10772178
- DOI: 10.1038/s41392-023-01688-x
Ischemia-reperfusion injury: molecular mechanisms and therapeutic targets
Abstract
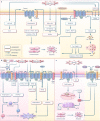
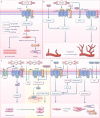
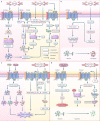
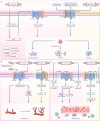
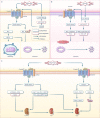
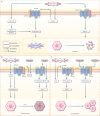
Ischemia-reperfusion (I/R) injury paradoxically occurs during reperfusion following ischemia, exacerbating the initial tissue damage. The limited understanding of the intricate mechanisms underlying I/R injury hinders the development of effective therapeutic interventions. The Wnt signaling pathway exhibits extensive crosstalk with various other pathways, forming a network system of signaling pathways involved in I/R injury. This review article elucidates the underlying mechanisms involved in Wnt signaling, as well as the complex interplay between Wnt and other pathways, including Notch, phosphatidylinositol 3-kinase/protein kinase B, transforming growth factor-β, nuclear factor kappa, bone morphogenetic protein, N-methyl-D-aspartic acid receptor-Ca2+-Activin A, Hippo-Yes-associated protein, toll-like receptor 4/toll-interleukine-1 receptor domain-containing adapter-inducing interferon-β, and hepatocyte growth factor/mesenchymal-epithelial transition factor. In particular, we delve into their respective contributions to key pathological processes, including apoptosis, the inflammatory response, oxidative stress, extracellular matrix remodeling, angiogenesis, cell hypertrophy, fibrosis, ferroptosis, neurogenesis, and blood-brain barrier damage during I/R injury. Our comprehensive analysis of the mechanisms involved in Wnt signaling during I/R reveals that activation of the canonical Wnt pathway promotes organ recovery, while activation of the non-canonical Wnt pathways exacerbates injury. Moreover, we explore novel therapeutic approaches based on these mechanistic findings, incorporating evidence from animal experiments, current standards, and clinical trials. The objective of this review is to provide deeper insights into the roles of Wnt and its crosstalk signaling pathways in I/R-mediated processes and organ dysfunction, to facilitate the development of innovative therapeutic agents for I/R injury.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

