Two rare variants that affect the same amino acid in CFTR have distinct responses to ivacaftor
- PMID: 38186087
- PMCID: PMC10872379
- DOI: 10.1113/JP285727
Two rare variants that affect the same amino acid in CFTR have distinct responses to ivacaftor
Abstract
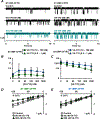
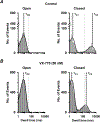
Some residues in the cystic fibrosis transmembrane conductance regulator (CFTR) channel are the site of more than one CFTR variant that cause cystic fibrosis. Here, we investigated the function of S1159F and S1159P, two variants associated with different clinical phenotypes, which affect the same pore-lining residue in transmembrane segment 12 that are both strongly potentiated by ivacaftor when expressed in CFBE41o- bronchial epithelial cells. To study the single-channel behaviour of CFTR, we applied the patch-clamp technique to Chinese hamster ovary cells heterologously expressing CFTR variants incubated at 27°C to enhance channel residence at the plasma membrane. S1159F- and S1159P-CFTR formed Cl- channels activated by cAMP-dependent phosphorylation and gated by ATP that exhibited thermostability at 37°C. Both variants modestly reduced the single-channel conductance of CFTR. By severely attenuating channel gating, S1159F- and S1159P-CFTR reduced the open probability (Po ) of wild-type CFTR by ≥75% at ATP (1 mM); S1159F-CFTR caused the greater decrease in Po consistent with its more severe clinical phenotype. Ivacaftor (10-100 nM) doubled the Po of both CFTR variants without restoring Po values to wild-type levels, but concomitantly, ivacaftor decreased current flow through open channels. For S1159F-CFTR, the reduction of current flow was marked at high (supersaturated) ivacaftor concentrations (0.5-1 μM) and voltage-independent, identifying an additional detrimental action of elevated ivacaftor concentrations. In conclusion, S1159F and S1159P are gating variants, which also affect CFTR processing and conduction, but not stability, necessitating the use of combinations of CFTR modulators to optimally restore their channel activity. KEY POINTS: Dysfunction of the ion channel cystic fibrosis transmembrane conductance regulator (CFTR) causes the genetic disease cystic fibrosis (CF). This study investigated two rare pathogenic CFTR variants, S1159F and S1159P, which affect the same amino acid in CFTR, to understand the molecular basis of disease and response to the CFTR-targeted therapy ivacaftor. Both rare variants diminished CFTR function by modestly reducing current flow through the channel and severely inhibiting ATP-dependent channel gating with S1159F exerting the stronger adverse effect, which correlates with its association with more severe disease. Ivacaftor potentiated channel gating by both rare variants without restoring their activity to wild-type levels, but concurrently reduced current flow through open channels, particularly those of S1159F-CFTR. Our data demonstrate that S1159F and S1159P cause CFTR dysfunction by multiple mechanisms that require combinations of CFTR-targeted therapies to fully restore channel function.
Keywords: CFTR chloride ion channel; CFTR inhibition; CFTR potentiation; cystic fibrosis; ivacaftor (VX-770); rare variant.
© 2024 The Authors. The Journal of Physiology © 2024 The Physiological Society.
Conflict of interest statement
Competing interests
The authors have no conflicts of interest to declare.
Figures
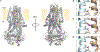
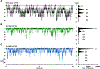
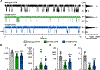

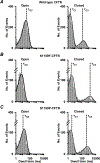



Similar articles
-
Corrector therapies (with or without potentiators) for people with cystic fibrosis with class II CFTR gene variants (most commonly F508del).Cochrane Database Syst Rev. 2023 Nov 20;11(11):CD010966. doi: 10.1002/14651858.CD010966.pub4. Cochrane Database Syst Rev. 2023. PMID: 37983082 Free PMC article.
-
Potentiators (specific therapies for class III and IV mutations) for cystic fibrosis.Cochrane Database Syst Rev. 2015 Mar 26;(3):CD009841. doi: 10.1002/14651858.CD009841.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2019 Jan 07;1:CD009841. doi: 10.1002/14651858.CD009841.pub3. PMID: 25811419 Updated.
-
Molecular and pharmacological evaluation of rare, cystic fibrosis-causing missense mutations of the CFTR channel.J Physiol. 2025 Aug 10. doi: 10.1113/JP288955. Online ahead of print. J Physiol. 2025. PMID: 40785054
-
Vanzacaftor-tezacaftor-deutivacaftor versus elexacaftor-tezacaftor-ivacaftor in individuals with cystic fibrosis aged 12 years and older (SKYLINE Trials VX20-121-102 and VX20-121-103): results from two randomised, active-controlled, phase 3 trials.Lancet Respir Med. 2025 Mar;13(3):256-271. doi: 10.1016/S2213-2600(24)00411-9. Epub 2025 Jan 2. Lancet Respir Med. 2025. PMID: 39756424 Free PMC article. Clinical Trial.
-
Evaluation of elexacaftor-tezacaftor-ivacaftor treatment in individuals with cystic fibrosis and CFTRN1303K in the USA: a prospective, multicentre, open-label, single-arm trial.Lancet Respir Med. 2024 Dec;12(12):947-957. doi: 10.1016/S2213-2600(24)00205-4. Epub 2024 Aug 26. Lancet Respir Med. 2024. PMID: 39208836
Cited by
-
Novel gain-of-function mutants identify a critical region within CFTR membrane-spanning domain 2 controlling cAMP-dependent and ATP-independent channel activation.Cell Mol Life Sci. 2024 Oct 7;81(1):426. doi: 10.1007/s00018-024-05431-9. Cell Mol Life Sci. 2024. PMID: 39373784 Free PMC article.
References
-
- Aleksandrov L, Aleksandrov AA, Chang X-B, & Riordan JR (2002). The first nucleotide binding domain of cystic fibrosis transmembrane conductance regulator is a site of stable nucleotide interaction, whereas the second is a site of rapid turnover. The Journal of Biological Chemistry 277, 15419–15425. - PubMed
-
- Anderson MP, & Welsh MJ (1992). Regulation by ATP and ADP of CFTR chloride channels that contain mutant nucleotide-binding domains. Science 257, 1701–1704. - PubMed
-
- Anderson MP, Berger HA, Rich DP, Gregory RJ, Smith AE, & Welsh MJ (1991). Nucleoside triphosphates are required to open the CFTR chloride channel. Cell 67, 775–784. - PubMed
-
- Bihler H, Sivachenko A, Millen L, Bhatt P, Thakerar Patel A, Chin J, Bailey V, Musisi I, LaPan A, Allaire NE, Conte J, Simon NR, Magaret AS, Raraigh KS, Cutting GR, Skach WR, Bridges RJ, Thomas PJ, & Mense M (2023). In vitro modulator responsiveness of 655 CFTR variants found in people with CF. bioRxiv DOI: 10.1101/2023.07.07.548159. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

