L-type amino acid transporter 1 inhibitor JPH203 prevents the growth of cabazitaxel-resistant prostate cancer by inhibiting cyclin-dependent kinase activity
- PMID: 38186218
- PMCID: PMC10920979
- DOI: 10.1111/cas.16062
L-type amino acid transporter 1 inhibitor JPH203 prevents the growth of cabazitaxel-resistant prostate cancer by inhibiting cyclin-dependent kinase activity
Abstract
L-type amino acid transporter 1 (LAT1, SLC7A5) is an amino acid transporter expressed in various carcinomas, and it is postulated to play an important role in the proliferation of cancer cells through the uptake of essential amino acids. Cabazitaxel is a widely used anticancer drug for treating castration-resistant prostate cancer (CRPC); however, its effectiveness is lost when cancer cells acquire drug resistance. In this study, we investigated the expression of LAT1 and the effects of a LAT1-specific inhibitor, JPH203, in cabazitaxel-resistant prostate cancer cells. LAT1 was more highly expressed in the cabazitaxel-resistant strains than in the normal strains. Administration of JPH203 inhibited the growth, migration, and invasive ability of cabazitaxel-resistant strains in vitro. Phosphoproteomics using liquid chromatography-mass spectrometry to comprehensively investigate changes in phosphorylation due to JPH203 administration revealed that cell cycle-related pathways were affected by JPH203, and that JPH203 significantly reduced the kinase activity of cyclin-dependent kinases 1 and 2. Moreover, JPH203 inhibited the proliferation of cabazitaxel-resistant cells in vivo. Taken together, the present study results suggest that LAT1 might be a valuable therapeutic target in cabazitaxel-resistant prostate cancer.
Keywords: CDK; LAT1; amino acid transporter; phosphoproteomics; prostate cancer.
© 2024 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
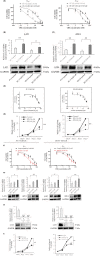

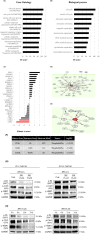
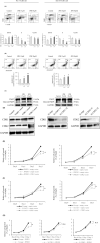
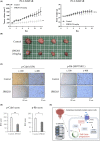
References
-
- Gandaglia G, Leni R, Bray F, et al. Epidemiology and prevention of prostate cancer. Eur Urol Oncol. 2021;4:877‐892. - PubMed
-
- Pobel C, Auclin E, Procureur A, et al. Cabazitaxel schedules in metastatic castration‐resistant prostate cancer: a review. Future Oncol. 2021;17:91‐102. - PubMed
-
- Galletti G, Leach BI, Lam L, Tagawa ST. Mechanisms of resistance to systemic therapy in metastatic castration‐resistant prostate cancer. Cancer Treat Rev. 2017;57:16‐27. - PubMed
-
- Miyao T, Koike H, Sekine Y, Ohtsu A, Oka D, Suzuki K. YM155 reverses Cabazitaxel resistance in castration‐resistant prostate cancer by reducing Survivin expression. Anticancer Res. 2020;40:5091‐5095. - PubMed
MeSH terms
Substances
Grants and funding
- #20K09555/Grants-in-Aid for Scientific Research of the Ministry of Education, Culture, Sports, Science and Technology of Japan
- #20H03813/Grants-in-Aid for Scientific Research of the Ministry of Education, Culture, Sports, Science and Technology of Japan
- #20K09572/Grants-in-Aid for Scientific Research of the Ministry of Education, Culture, Sports, Science and Technology of Japan
- #20K18087/Grants-in-Aid for Scientific Research of the Ministry of Education, Culture, Sports, Science and Technology of Japan
LinkOut - more resources
Full Text Sources
Medical
Research Materials

