Antimicrobial Effectiveness Testing of Resorbable Electrospun Fiber Matrix per United States Pharmacopeia (USP) <51>
- PMID: 38186476
- PMCID: PMC10768939
- DOI: 10.7759/cureus.50055
Antimicrobial Effectiveness Testing of Resorbable Electrospun Fiber Matrix per United States Pharmacopeia (USP) <51>
Abstract
Contamination of surgical, traumatic, and chronic wounds with microorganisms presents a challenge to successful wound healing. In the present in vitro study, a synthetic electrospun fiber matrix (SEFM) cleared for use in the management of chronic, surgical, and traumatic wounds underwent USP (United States Pharmacopeia) <51> Antimicrobial Effectiveness Testing to determine its in vitro effectiveness against various microorganisms commonly found in non-healing wounds. The SEFM was tested in both sheet (s-SEFM) and micronized form (m-SEFM) against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Aspergillus brasiliensis, Candida albicans, Proteus mirabilis, and Enterococcus faecalis. Testing was performed per the USP <51> standard on days 7, 14, and 28. Both the s-SEFM and m-SEFM met the USP <51> acceptance criteria for all microorganisms. The results obtained for s-SEFM demonstrated >1-log10 reduction against E. coli, S. aureus, P. aeruginosa, P. mirabilis, E. faecalis, and C. albicans at day 7; >3-log10 reduction with no detection of these microbes at days 14 and 28, and no increase from initial inoculum at days 7, 14, and 28 against A. brasiliensis. The results obtained for m-SEFM demonstrated >3-log10 reduction with no detectable microorganisms at day 7. The results observed in this study indicate that the SEFM is effective in vitro at inhibiting bacterial and fungal growth and colonization per USP <51> testing.
Keywords: contaminated wound; contamination; electrospinning; extracellular matrix; non-healing wound; surgical wound; synthetic electrospun fiber matrix; traumatic wound; usp <51> antimicrobial effectiveness testing; wound healing.
Copyright © 2023, Sallade et al.
Conflict of interest statement
Matthew MacEwan is the patent owner of the Restrata matrix
Figures


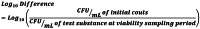

Similar articles
-
Synthetic Electrospun Fiber Matrix May Resemble Fungal Hyphae Over the Course of Tissue Regeneration: A Retrospective Review.Int J Surg Pathol. 2025 Apr;33(2):389-393. doi: 10.1177/10668969241265016. Epub 2024 Oct 29. Int J Surg Pathol. 2025. PMID: 39471849 Review.
-
The effect of a wound care solution containing polyhexanide and betaine on bacterial counts: results of an in vitro study .Ostomy Wound Manage. 2012 Oct;58(10):32-6. Ostomy Wound Manage. 2012. PMID: 23037330
-
Antimicrobial Blue Light Inactivation of Microbial Isolates in Biofilms.Lasers Surg Med. 2020 Jun;52(5):472-478. doi: 10.1002/lsm.23159. Epub 2019 Sep 19. Lasers Surg Med. 2020. PMID: 31536154 Free PMC article.
-
Synergistic clinical application of synthetic electrospun fiber wound matrix in the management of a complex traumatic wound: degloving left groin and thigh auger injury.Wounds. 2024 Apr;36(4):124-128. doi: 10.25270/wnds/23117. Wounds. 2024. PMID: 38743858
-
Sulopenem: An Intravenous and Oral Penem for the Treatment of Urinary Tract Infections Due to Multidrug-Resistant Bacteria.Drugs. 2022 Apr;82(5):533-557. doi: 10.1007/s40265-022-01688-1. Epub 2022 Mar 16. Drugs. 2022. PMID: 35294769 Review.
References
-
- Dowsett C, Ferreira F, Ousey K, Sandy-Hodgetts K, Romanelli M, Djohan R, Hurd T. Wounds International - a division of Omnia-Med Ltd. Hatfields, London, United Kingdom. Hatsfield, London: Wounds International; 2018. Surgical wound dehiscence: improving prevention and outcomes; pp. 1–47.
-
- Back to basics: preventing surgical site infections. Spruce L. AORN J. 2014;99:600–608. - PubMed
-
- Risk factors for surgical site infection. Cheadle WG. Surg Infect (Larchmt) 2006;7 Suppl 1:0–11. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
