Survival benefit of metastasectomy in first-line cetuximab therapy in patients with RAS wild-type metastatic colorectal cancer: a nationwide registry
- PMID: 38187069
- PMCID: PMC10767339
Survival benefit of metastasectomy in first-line cetuximab therapy in patients with RAS wild-type metastatic colorectal cancer: a nationwide registry
Abstract
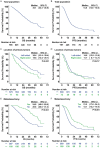
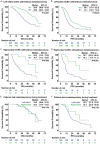
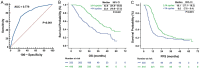
This multicenter study aimed to explore the survival benefit of metastasectomy by first-line cetuximab-based chemotherapy in real-world patients with RAS wild-type metastatic colorectal cancer (mCRC). The primary endpoints were overall survival (OS) and progression-free survival (PFS). The secondary endpoints included objective response rate (ORR), disease control rate (DCR), and metastasectomy rate. The exploratory endpoint was the optimal treatment cycle for better OS and PFS. Receiver operating characteristic curve with the area under curve (AUC) was used to identify the optimal cut-off cycle for survival outcomes. A total of 758 mCRC patients were enrolled in this study, with a median OS of 35.1 months, median PFS of 14.6 months, and metastasectomy rate of 21.4%. Left-sided mCRC had a significantly higher DCR (88.9% vs. 73.1%, P<0.001) and better OS (36.4 vs. 19.6 months, P<0.001). There were no significant differences in PFS and metastasectomy rate between left-sided and right-sided mCRC. However, mCRC patients who underwent metastasectomy over the course of treatment had better OS (54.9 vs. 28.6 months, P<0.001) and PFS (21.0 vs. 13.1 months, P<0.001) than those who did not. Notably, right-sided mCRC who benefited from first-line cetuximab-based chemotherapy to underwent metastasectomy also had favorable outcomes, on a par with left-sided mCRC. The optimal treatment cycle was 14 cycles (AUC: 0.779, P<0.001). Patients who received ≥14 cycles had higher metastasectomy rates (27.5% vs. 13.5%, P<0.001), favorable OS (42.6 vs. 23.4 months, P<0.001) and PFS (18.1 vs. 8.6 months, P<0.001), and, importantly, had comparable adverse events compared with patients who received <14 cycles of treatment. Patients who underwent metastasectomy after or during first-line cetuximab therapy have an improved OS in both left-sided and right-sided mCRC. Furthermore, patients receive ≥14 cycles of treatment whenever possible to achieve a higher likelihood of metastasectomy was associated with favorable survival outcomes.
Keywords: RAS wild-type metastatic colorectal cancer; cetuximab; metastasectomy; optimal treatment cycle; overall survival; progression-free survival.
AJCR Copyright © 2023.
Conflict of interest statement
None.
Figures
Similar articles
-
Patients with RAS wild-type right-sided unresectable liver-confined mCRC also benefit from cetuximab plus chemotherapy in first-line treatment.Am J Cancer Res. 2018 Nov 1;8(11):2337-2345. eCollection 2018. Am J Cancer Res. 2018. PMID: 30555748 Free PMC article.
-
An Exosome-Based Liquid Biopsy Predicts Depth of Response and Survival Outcomes to Cetuximab and Panitumumab in Metastatic Colorectal Cancer: The EXONERATE Study.Clin Cancer Res. 2025 Mar 17;31(6):1002-1015. doi: 10.1158/1078-0432.CCR-24-1934. Clin Cancer Res. 2025. PMID: 39820673
-
FOLFOX plus anti-epidermal growth factor receptor (EGFR) monoclonal antibody (mAb) is an effective first-line treatment for patients with RAS-wild left-sided metastatic colorectal cancer: A meta-analysis.Medicine (Baltimore). 2018 Mar;97(10):e0097. doi: 10.1097/MD.0000000000010097. Medicine (Baltimore). 2018. PMID: 29517682 Free PMC article. Review.
-
Phase II APEC trial: The impact of primary tumor side on outcomes of first-line cetuximab plus FOLFOX or FOLFIRI in patients with RAS wild-type metastatic colorectal cancer.Asia Pac J Clin Oncol. 2019 Aug;15(4):225-230. doi: 10.1111/ajco.13154. Epub 2019 May 15. Asia Pac J Clin Oncol. 2019. PMID: 31090260 Free PMC article. Clinical Trial.
-
Primary tumour side as a driver for treatment choice in RAS wild-type metastatic colorectal cancer patients: a systematic review and pooled analysis of randomised trials.Eur J Cancer. 2023 May;184:106-116. doi: 10.1016/j.ejca.2023.02.006. Epub 2023 Feb 17. Eur J Cancer. 2023. PMID: 36913832
Cited by
-
Outcomes of upfront primary tumor resection in patients with synchronous RAS wild-type metastatic colorectal cancer.Am J Cancer Res. 2024 Dec 15;14(12):5863-5873. doi: 10.62347/DLWI1455. eCollection 2024. Am J Cancer Res. 2024. PMID: 39803657 Free PMC article.
-
Impact on survival benefits of asymptomatic primary tumor resection after bevacizumab plus FOLFIRI as first-line therapy for patients with metastatic colorectal cancer with synchronous unresectable metastasis.Int J Colorectal Dis. 2024 Oct 25;39(1):171. doi: 10.1007/s00384-024-04745-1. Int J Colorectal Dis. 2024. PMID: 39453531 Free PMC article.
-
Efficacy and safety of first-line cetuximab therapy for older patients (aged ≥ 70 years) with RAS wild-type metastatic colorectal cancer: a nationwide real-world study.Am J Cancer Res. 2025 Jul 15;15(7):3093-3105. doi: 10.62347/QZFD8646. eCollection 2025. Am J Cancer Res. 2025. PMID: 40814368 Free PMC article.
-
Prognostic impact of metastatic sites and its metastasectomy in colorectal cancer: a retrospective analysis from a single institution.Int J Colorectal Dis. 2025 Jul 2;40(1):150. doi: 10.1007/s00384-025-04943-5. Int J Colorectal Dis. 2025. PMID: 40603598 Free PMC article.
References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:329–359. - PubMed
-
- Benson AB, Venook AP, Al-Hawary MM, Azad N, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Jeck W, Johung KL, Kirilcuk N, Krishnamurthi S, Maratt JK, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stotsky-Himelfarb E, Tavakkoli A, Willett CG, Gregory K, Gurski L. Rectal cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:1139–1167. - PubMed
-
- Zarour LR, Anand S, Billingsley KG, Bisson WH, Cercek A, Clarke MF, Coussens LM, Gast CE, Geltzeiler CB, Hansen L, Kelley KA, Lopez CD, Rana SR, Ruhl R, Tsikitis VL, Vaccaro GM, Wong MH, Mayo SC. Colorectal cancer liver metastasis: evolving paradigms and future directions. Cell Mol Gastroenterol Hepatol. 2017;3:163–173. - PMC - PubMed
-
- Cremolini C, Antoniotti C, Moretto R, Masi G, Falcone A. First-line therapy for mCRC - the influence of primary tumour location on the therapeutic algorithm. Nat Rev Clin Oncol. 2017;14:113. - PubMed
LinkOut - more resources
Full Text Sources
