Teplizumab in Type 1 Diabetes Mellitus: An Updated Review
- PMID: 38187075
- PMCID: PMC10769466
- DOI: 10.17925/EE.2023.19.2.7
Teplizumab in Type 1 Diabetes Mellitus: An Updated Review
Abstract
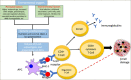
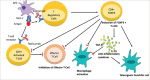
Type 1 diabetes mellitus (T1DM) is a chronic autoimmune condition characterized by the irreversible destruction of the β cells of the pancreas, which leads to a lifelong dependency on exogenous insulin. Despite the advancements in insulin delivery methods, the suboptimal outcomes of these methods have triggered the search for therapies that may prevent or reverse the disease. Given the autoimmune aetiology of T1DM, therapies counteracting the immune-mediated destruction of the β-cells are the obvious target. Although several treatment strategies have been attempted to target cellular, humoral and innate immunity, very few have had a clinically meaningful impact. Of all the available immunomodulatory agents, cluster of differentiation (CD) 3 antibodies have exhibited the most promising preclinical and clinical results. Muromonab-CD3, which also happened to be a murine CD3 antibody, was the first monoclonal antibody approved for clinical use and was primarily indicated for graft rejection. The adverse effects associated with muromonab-CD3 led to its withdrawal. Teplizumab, a newer CD3 antibody, has a better side-effect profile because of its humanized nature and non-Fc-receptor-binding domain. In November 2022, teplizumab became the first immunomodulatory agent to be licensed by the US Food and Drug Administration for delaying the onset of T1DM in high-risk adults and children over 8 years old. The mechanism seems to be enhancing regulatory T-cell activity and promoting immune tolerance. This article reviews the mechanism of action and the clinical trials of teplizumab in individuals with T1DM or at risk of developing the disease.
Keywords: Anti-CD3 monoclonal antibody; immunomodulation; regulatory T cells; teplizumab; tolerance; type 1 diabetes mellitus.
© Touch Medical Media 2023.
Conflict of interest statement
Disclosures: Simran Thakkar, Aditi Chopra, Lakshmi Nagendra, Sanjay Kalra and Saptarshi Bhattacharya have no financial or non-financial relationships or activities to declare in relation to this article.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources