Single-cell immunophenotyping revealed the association of CD4+ central and CD4+ effector memory T cells linking exacerbating chronic obstructive pulmonary disease and NSCLC
- PMID: 38187374
- PMCID: PMC10770259
- DOI: 10.3389/fimmu.2023.1297577
Single-cell immunophenotyping revealed the association of CD4+ central and CD4+ effector memory T cells linking exacerbating chronic obstructive pulmonary disease and NSCLC
Abstract
Introduction: Tobacco smoking generates airway inflammation in chronic obstructive pulmonary disease (COPD), and its involvement in the development of lung cancer is still among the leading causes of early death. Therefore, we aimed to have a better understanding of the disbalance in immunoregulation in chronic inflammatory conditions in smoker subjects with stable COPD (stCOPD), exacerbating COPD (exCOPD), or non-small cell lung cancer (NSCLC).
Methods: Smoker controls without chronic illness were recruited as controls. Through extensive mapping of single cells, surface receptor quantification was achieved by single-cell mass cytometry (CyTOF) with 29 antibodies. The CyTOF characterized 14 main immune subsets such as CD4+, CD8+, CD4+/CD8+, CD4-/CD8-, and γ/δ T cells and other subsets such as CD4+ or CD8+ NKT cells, NK cells, B cells, plasmablasts, monocytes, CD11cdim, mDCs, and pDCs. The CD4+ central memory (CM) T cells (CD4+/CD45RA-/CD45RO+/CD197+) and CD4+ effector memory (EM) T cells (CD4+/CD45RA-/CD45RO+/CD197-) were FACS-sorted for RNA-Seq analysis. Plasma samples were assayed by Luminex MAGPIX® for the quantitative measurement of 17 soluble immuno-oncology mediators (BTLA, CD28, CD80, CD27, CD40, CD86, CTLA-4, GITR, GITRL, HVEM, ICOS, LAG-3, PD-1, PD-L1, PD-L2, TIM-3, TLR-2) in the four studied groups.
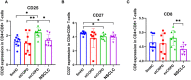
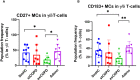
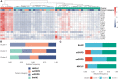
Results: Our focus was on T-cell-dependent differences in COPD and NSCLC, where peripheral CD4+ central memory and CD4+ effector memory cells showed a significant reduction in exCOPD and CD4+ CM showed elevation in NSCLC. The transcriptome analysis delineated a perfect correlation of differentially expressed genes between exacerbating COPD and NSCLC-derived peripheral CD4+ CM or CD4+ EM cells. The measurement of 17 immuno-oncology soluble mediators revealed a disease-associated phenotype in the peripheral blood of stCOPD, exCOPD, and NSCLC patients.
Discussion: The applied single-cell mass cytometry, the whole transcriptome profiling of peripheral CD4+ memory cells, and the quantification of 17 plasma mediators provided complex data that may contribute to the understanding of the disbalance in immune homeostasis generated or sustained by tobacco smoking in COPD and NSCLC.
Keywords: CD4 central memory T cells; CD4 effector memory T cells; exacerbating COPD; non-small cell lung cancer; single-cell mass cytometry; stable COPD; tobacco smoking.
Copyright © 2023 Gémes, Balog, Neuperger, Schlegl, Barta, Fillinger, Antus, Zvara, Hegedűs, Czimmerer, Manczinger, Balogh, Tóvári, Puskás and Szebeni.
Conflict of interest statement
Author LP was the CEO of the company Avicor Ltd and Avidin Ltd. Author GS was employed by the company CS-Smartlab Devices Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Figures






Comment on
-
Systemic alterations in neutrophils and their precursors in early-stage chronic obstructive pulmonary disease.Cell Rep. 2023 Jun 27;42(6):112525. doi: 10.1016/j.celrep.2023.112525. Epub 2023 May 26. Cell Rep. 2023. PMID: 37243592 Free PMC article.
References
-
- Safiri S, Carson-Chahhoud K, Noori M, Nejadghaderi SA, Sullman MJM, Ahmadian Heris J, et al. . Burden of chronic obstructive pulmonary disease and its attributable risk factors in 204 countries and territories, 1990-2019: results from the Global Burden of Disease Study 2019. BMJ (2022) 378:e069679. doi: 10.1136/bmj-2021-069679 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

