A novel technique for delineating the effect of variation in the learning rate on the neural correlates of reward prediction errors in model-based fMRI
- PMID: 38187436
- PMCID: PMC10768009
- DOI: 10.3389/fpsyg.2023.1211528
A novel technique for delineating the effect of variation in the learning rate on the neural correlates of reward prediction errors in model-based fMRI
Abstract
Introduction: Computational models play an increasingly important role in describing variation in neural activation in human neuroimaging experiments, including evaluating individual differences in the context of psychiatric neuroimaging. In particular, reinforcement learning (RL) techniques have been widely adopted to examine neural responses to reward prediction errors and stimulus or action values, and how these might vary as a function of clinical status. However, there is a lack of consensus around the importance of the precision of free parameter estimation for these methods, particularly with regard to the learning rate. In the present study, I introduce a novel technique which may be used within a general linear model (GLM) to model the effect of mis-estimation of the learning rate on reward prediction error (RPE)-related neural responses.
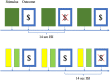
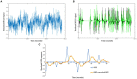
Methods: Simulations employed a simple RL algorithm, which was used to generate hypothetical neural activations that would be expected to be observed in functional magnetic resonance imaging (fMRI) studies of RL. Similar RL models were incorporated within a GLM-based analysis method including derivatives, with individual differences in the resulting GLM-derived beta parameters being evaluated with respect to the free parameters of the RL model or being submitted to other validation analyses.
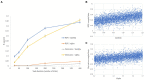
Results: Initial simulations demonstrated that the conventional approach to fitting RL models to RPE responses is more likely to reflect individual differences in a reinforcement efficacy construct (lambda) rather than learning rate (alpha). The proposed method, adding a derivative regressor to the GLM, provides a second regressor which reflects the learning rate. Validation analyses were performed including examining another comparable method which yielded highly similar results, and a demonstration of sensitivity of the method in presence of fMRI-like noise.
Conclusion: Overall, the findings underscore the importance of the lambda parameter for interpreting individual differences in RPE-coupled neural activity, and validate a novel neural metric of the modulation of such activity by individual differences in the learning rate. The method is expected to find application in understanding aberrant reinforcement learning across different psychiatric patient groups including major depression and substance use disorder.
Keywords: fMRI; general linear model; prediction errors; reinforcement learning; reinforcement sensitivity.
Copyright © 2023 Chase.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Working Memory Load Strengthens Reward Prediction Errors.J Neurosci. 2017 Apr 19;37(16):4332-4342. doi: 10.1523/JNEUROSCI.2700-16.2017. Epub 2017 Mar 20. J Neurosci. 2017. PMID: 28320846 Free PMC article.
-
Reward Learning as a Potential Mechanism for Improvement in Schizophrenia Spectrum Disorders Following Cognitive Remediation: Protocol for a Clinical, Nonrandomized, Pre-Post Pilot Study.JMIR Res Protoc. 2024 Jan 22;13:e52505. doi: 10.2196/52505. JMIR Res Protoc. 2024. PMID: 38252470 Free PMC article.
-
Probing relationships between reinforcement learning and simple behavioral strategies to understand probabilistic reward learning.J Neurosci Methods. 2020 Jul 15;341:108777. doi: 10.1016/j.jneumeth.2020.108777. Epub 2020 May 15. J Neurosci Methods. 2020. PMID: 32417532
-
Prediction error in reinforcement learning: a meta-analysis of neuroimaging studies.Neurosci Biobehav Rev. 2013 Aug;37(7):1297-310. doi: 10.1016/j.neubiorev.2013.03.023. Epub 2013 Apr 6. Neurosci Biobehav Rev. 2013. PMID: 23567522 Review.
-
Reinforcement learning in depression: A review of computational research.Neurosci Biobehav Rev. 2015 Aug;55:247-67. doi: 10.1016/j.neubiorev.2015.05.005. Epub 2015 May 12. Neurosci Biobehav Rev. 2015. PMID: 25979140 Review.
References
LinkOut - more resources
Full Text Sources

