Pyrrolo[2,1- a]isoquinoline scaffolds for developing anti-cancer agents
- PMID: 38187449
- PMCID: PMC10768717
- DOI: 10.1039/d3ra07047f
Pyrrolo[2,1- a]isoquinoline scaffolds for developing anti-cancer agents
Abstract
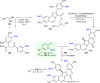
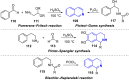
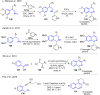
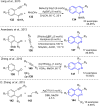
Fused pyrrolo[2,1-a]isoquinolines have emerged as compelling molecules with remarkably potent cytotoxic activity and topoisomerase inhibitors. This comprehensive review delves into the intricate world of this family of compounds, analyzing the natural marine lamellarins known for their diverse and complex chemical structures, exploring structure-activity relationships (SARs), and highlighting their remarkable versatility. The review emphasizes their fundamental role as topoisomerase inhibitors and cytotoxic agents, as well as some crucial aspects of the chemistry of pyrrolo[2,1-a]isoquinolines, exploring synthetic strategies in total synthesis and molecular diversification trends, highlighting their importance in the field of medicinal chemistry and beyond.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare nonfinancial interests/personal relationships, which may be considered as potential competing interests.
Figures





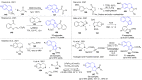
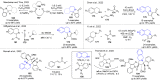
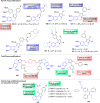
References
-
- Fukuda T. Nanjo Y. Fujimoto M. Yoshida K. Natsui Y. Ishibashi F. Okazaki F. To H. Iwao M. Bioorganic Med. Chem. 2019;27:265–277. - PubMed
-
- Fukuda T., Ishibashi F. and Iwao M., Lamellarin Alkaloids: Isolation, Synthesis, and Biological Activity, Elsevier Inc., 1st edn, 2020, vol. 83 - PubMed
-
- Zheng L. Gao T. Ge Z. Ma Z. Xu J. Ding W. Shen L. Eur. J. Med. Chem. 2021;214:113226. - PubMed
-
- Chittchang M. Paul Gleeson M. Ploypradith P. Ruchirawat S. Eur. J. Med. Chem. 2010;45:2165–2172. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

