Drug targets regulate systemic metabolism and provide new horizons to treat nonalcoholic steatohepatitis
- PMID: 38187470
- PMCID: PMC10770762
- DOI: 10.1016/j.metop.2023.100267
Drug targets regulate systemic metabolism and provide new horizons to treat nonalcoholic steatohepatitis
Abstract
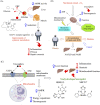
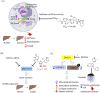
Nonalcoholic steatohepatitis (NASH), is the advanced stage of nonalcoholic fatty liver disease (NAFLD) with rapidly rising global prevalence. It is featured with severe hepatocyte apoptosis, inflammation and hepatic lipogenesis. The drugs directly targeting the processes of steatosis, inflammation and fibrosis are currently under clinical investigation. Nevertheless, the long-term ineffectiveness and remarkable adverse effects are well documented, and new concepts are required to tackle with the root causes of NASH progression. We critically assess the recently validated drug targets that regulate the systemic metabolism to ameliorate NASH. Thermogenesis promoted by mitochondrial uncouplers restores systemic energy expenditure. Furthermore, regulation of mitochondrial proteases and proteins that are pivotal for intracellular metabolic homeostasis normalize mitochondrial function. Secreted proteins also improve systemic metabolism, and NASH is ameliorated by agonizing receptors of secreted proteins with small molecules. We analyze the drug design, the advantages and shortcomings of these novel drug candidates. Meanwhile, the structural modification of current NASH therapeutics significantly increased their selectivity, efficacy and safety. Furthermore, the arising CRISPR-Cas9 screen strategy on liver organoids has enabled the identification of new genes that mediate lipid metabolism, which may serve as promising drug targets. In summary, this article discusses the in-depth novel mechanisms and the multidisciplinary approaches, and they provide new horizons to treat NASH.
Keywords: Drug discovery; Drug targets; Energy expenditure; Mechanisms; Metabolic homeostasis; NASH.
© 2023 The Authors. Published by Elsevier Inc.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Direct degradation and stabilization of proteins: New horizons in treatment of nonalcoholic steatohepatitis.Biochem Pharmacol. 2024 Feb;220:115989. doi: 10.1016/j.bcp.2023.115989. Epub 2023 Dec 18. Biochem Pharmacol. 2024. PMID: 38122854 Review.
-
Emerging and Established Therapeutic Approaches for Nonalcoholic Fatty Liver Disease.Clin Ther. 2021 Sep;43(9):1476-1504. doi: 10.1016/j.clinthera.2021.07.013. Epub 2021 Aug 24. Clin Ther. 2021. PMID: 34446271 Review.
-
Liraglutide improves hepatic steatosis and metabolic dysfunctions in a 3-week dietary mouse model of nonalcoholic steatohepatitis.Am J Physiol Gastrointest Liver Physiol. 2019 Oct 1;317(4):G508-G517. doi: 10.1152/ajpgi.00139.2019. Epub 2019 Aug 28. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 31460789
-
Targeting MRG15 for the treatment of nonalcoholic steatohepatitis.Metabol Open. 2022 Nov 17;16:100217. doi: 10.1016/j.metop.2022.100217. eCollection 2022 Dec. Metabol Open. 2022. PMID: 36478775 Free PMC article.
-
Bile acid receptors and nonalcoholic fatty liver disease.World J Hepatol. 2015 Dec 8;7(28):2811-8. doi: 10.4254/wjh.v7.i28.2811. World J Hepatol. 2015. PMID: 26668692 Free PMC article. Review.
References
-
- Sheka A.C., Adeyi O., Thompson J., Hameed B., Crawford P.A., Ikramuddin S. Nonalcoholic steatohepatitis: a review. JAMA. 2020;323:1175–1183. - PubMed
-
- Wong R.J., Aguilar M., Cheung R., Perumpail R.B., Harrison S.A., Younossi Z.M., Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. - PubMed
-
- Lonardo A., Mantovani A., Petta S., Carraro A., Byrne C.D., Targher G. Metabolic mechanisms for and treatment of NAFLD or NASH occurring after liver transplantation. Nat Rev Endocrinol. 2022;18:638–650. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

