This is a preprint.
EGFR-dependent actomyosin patterning coordinates morphogenetic movements between tissues
- PMID: 38187543
- PMCID: PMC10769333
- DOI: 10.1101/2023.12.22.573057
EGFR-dependent actomyosin patterning coordinates morphogenetic movements between tissues
Update in
-
EGFR-dependent actomyosin patterning coordinates morphogenetic movements between tissues in Drosophila melanogaster.Dev Cell. 2025 Jan 20;60(2):270-287.e6. doi: 10.1016/j.devcel.2024.10.002. Epub 2024 Oct 25. Dev Cell. 2025. PMID: 39461341
Abstract
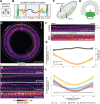
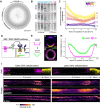
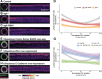
The movements that give rise to the body's structure are powered by cell shape changes and rearrangements that are coordinated at supracellular scales. How such cellular coordination arises and integrates different morphogenetic programs is unclear. Using quantitative imaging, we found a complex pattern of adherens junction (AJ) levels in the ectoderm prior to gastrulation onset in Drosophila. AJ intensity exhibited a double-sided gradient, with peaks at the dorsal midline and ventral neuroectoderm. We show that this dorsal-ventral AJ pattern is regulated by epidermal growth factor (EGF) signaling and that this signal is required for ectoderm cell movement during mesoderm invagination and axis extension. We identify AJ levels and junctional actomyosin as downstream effectors of EGFR signaling. Overall, our study demonstrates a mechanism of coordination between tissue folding and convergent extension that facilitates embryo-wide gastrulation movements.
Figures



Similar articles
-
EGFR-dependent actomyosin patterning coordinates morphogenetic movements between tissues in Drosophila melanogaster.Dev Cell. 2025 Jan 20;60(2):270-287.e6. doi: 10.1016/j.devcel.2024.10.002. Epub 2024 Oct 25. Dev Cell. 2025. PMID: 39461341
-
A contractile actomyosin network linked to adherens junctions by Canoe/afadin helps drive convergent extension.Mol Biol Cell. 2011 Jul 15;22(14):2491-508. doi: 10.1091/mbc.E11-05-0411. Epub 2011 May 25. Mol Biol Cell. 2011. PMID: 21613546 Free PMC article.
-
The Physical Mechanisms of Drosophila Gastrulation: Mesoderm and Endoderm Invagination.Genetics. 2020 Mar;214(3):543-560. doi: 10.1534/genetics.119.301292. Genetics. 2020. PMID: 32132154 Free PMC article.
-
Cellular and molecular mechanisms of convergence and extension in zebrafish.Curr Top Dev Biol. 2020;136:377-407. doi: 10.1016/bs.ctdb.2019.08.001. Epub 2019 Sep 3. Curr Top Dev Biol. 2020. PMID: 31959296 Free PMC article. Review.
-
The cellular and molecular mechanisms that establish the mechanics of Drosophila gastrulation.Curr Top Dev Biol. 2020;136:141-165. doi: 10.1016/bs.ctdb.2019.08.003. Epub 2019 Sep 13. Curr Top Dev Biol. 2020. PMID: 31959286 Review.
References
-
- Thompson D. (1917). On Growth and Form 1st ed. (Cambridge University Press; ).
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
