This is a preprint.
Cyclin A/Cdk1 promotes chromosome alignment and timely mitotic progression
- PMID: 38187612
- PMCID: PMC10769330
- DOI: 10.1101/2023.12.21.572788
Cyclin A/Cdk1 promotes chromosome alignment and timely mitotic progression
Update in
-
Cyclin A/Cdk1 promotes chromosome alignment and timely mitotic progression.Mol Biol Cell. 2024 Nov 1;35(11):ar141. doi: 10.1091/mbc.E23-12-0479. Epub 2024 Oct 2. Mol Biol Cell. 2024. PMID: 39356777 Free PMC article.
Abstract
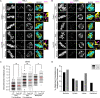
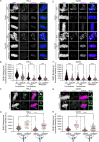
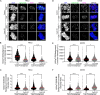
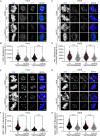
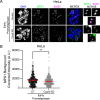
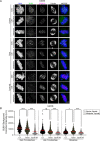
To ensure genomic fidelity a series of spatially and temporally coordinated events are executed during prometaphase of mitosis, including bipolar spindle formation, chromosome attachment to spindle microtubules at kinetochores, the correction of erroneous kinetochore-microtubule (k-MT) attachments, and chromosome congression to the spindle equator. Cyclin A/Cdk1 kinase plays a key role in destabilizing k-MT attachments during prometaphase to promote correction of erroneous k-MT attachments. However, it is unknown if Cyclin A/Cdk1 kinase regulates other events during prometaphase. Here, we investigate additional roles of Cyclin A/Cdk1 in prometaphase by using an siRNA knockdown strategy to deplete endogenous Cyclin A from human cells. We find that depleting Cyclin A significantly extends mitotic duration, specifically prometaphase, because chromosome alignment is delayed. Unaligned chromosomes display erroneous monotelic, syntelic, or lateral k-MT attachments suggesting that bioriented k-MT attachment formation is delayed in the absence of Cyclin A. Mechanistically, chromosome alignment is likely impaired because the localization of the kinetochore proteins BUB1 kinase, KNL1, and MPS1 kinase are reduced in Cyclin A-depleted cells. Moreover, we find that Cyclin A promotes BUB1 kinetochore localization independently of its role in destabilizing k-MT attachments. Thus, Cyclin A/Cdk1 facilitates chromosome alignment during prometaphase to support timely mitotic progression.
Figures


References
-
- Bodor DL, Rodríguez MG, Moreno N, and Jansen LET (2012). Analysis of Protein Turnover by Quantitative SNAP-Based Pulse-Chase Imaging. Curr Protoc Cell Biology 55, 8.8.1–8.8.34. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
