This is a preprint.
An AAV capsid reprogrammed to bind human Transferrin Receptor mediates brain-wide gene delivery
- PMID: 38187643
- PMCID: PMC10769326
- DOI: 10.1101/2023.12.20.572615
An AAV capsid reprogrammed to bind human Transferrin Receptor mediates brain-wide gene delivery
Update in
-
An AAV capsid reprogrammed to bind human transferrin receptor mediates brain-wide gene delivery.Science. 2024 Jun 14;384(6701):1220-1227. doi: 10.1126/science.adm8386. Epub 2024 May 16. Science. 2024. PMID: 38753766
Abstract
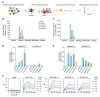
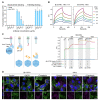
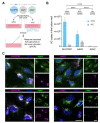
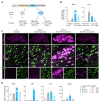
Developing vehicles that efficiently deliver genes throughout the human central nervous system (CNS) will broaden the range of treatable genetic diseases. We engineered an AAV capsid, BI-hTFR1, that binds human Transferrin Receptor (TfR1), a protein expressed on the blood-brain barrier (BBB). BI-hTFR1 was actively transported across a human brain endothelial cell layer and, relative to AAV9, provided 40-50 times greater reporter expression in the CNS of human TFRC knock-in mice. The enhanced tropism was CNS-specific and absent in wild type mice. When used to deliver GBA1, mutations of which cause Gaucher disease and are linked to Parkinson's disease, BI-hTFR1 substantially increased brain and cerebrospinal fluid glucocerebrosidase activity compared to AAV9. These findings establish BI-hTFR1 as a promising vector for human CNS gene therapy.
Conflict of interest statement
Competing interests B.E.D is a scientific founder and scientific advisory board member at Apertura Gene Therapy and a scientific advisory board member at Tevard Biosciences. B.E.D., A.J.B., K.Y.C., F.E.E., Q.H., J.W., and N.R.B.R. are named inventors on patent applications filed by the Broad Institute of MIT and Harvard related to this study. Remaining authors declare that they have no competing interests.
Figures


References
-
- Müller O. J., Kaul F., Weitzman M. D., Pasqualini R., Arap W., Kleinschmidt J. A., Trepel M., Random peptide libraries displayed on adeno-associated virus to select for targeted gene therapy vectors. Nat. Biotechnol. 21, 1040–1046 (2003). - PubMed
-
- Koerber J. T., Maheshri N., Kaspar B. K., Schaffer D. V., Construction of diverse adeno-associated viral libraries for directed evolution of enhanced gene delivery vehicles. Nat. Protoc. 1, 701–706 (2006). - PubMed
-
- Li W., Asokan A., Wu Z., Van Dyke T., DiPrimio N., Johnson J. S., Govindaswamy L., Agbandje-McKenna M., Leichtle S., Eugene Redmond D. Jr, McCown T. J., Petermann K. B., Sharpless N. E., Samulski R. J., Engineering and Selection of Shuffled AAV Genomes: A New Strategy for Producing Targeted Biological Nanoparticles. Mol. Ther. 16, 1252–1260 (2008). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
